|
Transmembrane
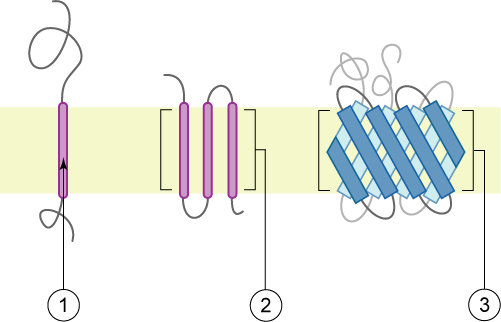
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Domain
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an alpha helix topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in length, sequence, and hydrophobicity, adopting organelle-specific properties. Functions of transmembrane domains Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across biological membranes; usually hydrophilic residues and binding sites in the TMDs help in this process. *Signal transduction across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Integral Membrane Protein
An integral, or intrinsic, membrane protein (IMP) is a type of membrane protein that is permanently attached to the biological membrane. All ''transmembrane proteins'' are IMPs, but not all IMPs are transmembrane proteins. IMPs comprise a significant fraction of the proteins encoded in an organism's genome. Proteins that cross the membrane are surrounded by annular lipids, which are defined as lipids that are in direct contact with a membrane protein. Such proteins can only be separated from the membranes by using detergents, nonpolar solvents, or sometimes denaturing agents. Structure Three-dimensional structures of ~160 different integral membrane proteins have been determined at atomic resolution by X-ray crystallography or nuclear magnetic resonance spectroscopy. They are challenging subjects for study owing to the difficulties associated with extraction and crystallization. In addition, structures of many water-soluble protein domains of IMPs are available in the Prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bitopic Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. A 2013 estimate identified about 1300 single-pass membrane proteins in the human genome. Topology-based classification Bitopic proteins are classified into 4 types, depending on their transmembrane topology and location of the transmembrane heli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bitopic Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to the network of interactions between different proteins in cells, including interactions via transmembrane alpha helices. They usually include one or several water-soluble domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. A 2013 estimate identified about 1300 single-pass membrane proteins in the human genome. Topology-based classification Bitopic proteins are classified into 4 types, depending on their transmembrane topology and location of the transmembrane heli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydropathy Plot
Hydrophobicity scales are values that define the relative hydrophobicity or hydrophilicity of amino acid residues. The more positive the value, the more hydrophobic are the amino acids located in that region of the protein. These scales are commonly used to predict the transmembrane alpha-helices of membrane proteins. When consecutively measuring amino acids of a protein, changes in value indicate attraction of specific protein regions towards the hydrophobic region inside lipid bilayer. The hydrophobic or hydrophilic character of a compound or amino acid is its hydropathic character, hydropathicity, or hydropathy. Hydrophobicity and the hydrophobic effect The hydrophobic effect represents the tendency of water to exclude non-polar molecules. The effect originates from the disruption of highly dynamic hydrogen bonds between molecules of liquid water. Polar chemical groups, such as OH group in methanol do not cause the hydrophobic effect. However, a pure hydrocarbon molecule, for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lipoproteins
A lipoprotein is a biochemical assembly whose primary function is to transport hydrophobic lipid (also known as fat) molecules in water, as in blood plasma or other extracellular fluids. They consist of a triglyceride and cholesterol center, surrounded by a phospholipid outer shell, with the hydrophilic portions oriented outward toward the surrounding water and lipophilic portions oriented inward toward the lipid center. A special kind of protein, called apolipoprotein, is embedded in the outer shell, both stabilising the complex and giving it a functional identity that determines its role. Many enzymes, transporters, structural proteins, antigens, adhesins, and toxins are lipoproteins. Examples include plasma lipoprotein particles ( HDL, LDL, IDL, VLDL and chylomicrons). Subgroups of these plasma particles are primary drivers or modulators of atherosclerosis. Scope Transmembrane lipoproteins Some transmembrane proteolipids, especially those found in bacteria, are referred t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyproline Helix
A polyproline helix is a type of protein secondary structure which occurs in proteins comprising repeating proline residues. A left-handed polyproline II helix (PPII, poly-Pro II) is formed when sequential residues all adopt (φ,ψ) backbone dihedral angles of roughly (-75°, 150°) and have ''Cis–trans isomerism, trans'' isomers of their peptide bonds. This PPII conformation is also common in proteins and polypeptides with other amino acids apart from proline. Similarly, a more compact right-handed polyproline I helix (PPI, poly-Pro I) is formed when sequential residues all adopt (φ,ψ) backbone dihedral angles of roughly (-75°, 160°) and have ''Cis–trans isomerism, cis'' isomers of their peptide bonds. Of the twenty common naturally occurring amino acids, only proline is likely to adopt the ''cis'' isomer of the peptide bond, specifically the X-Pro peptide bond; steric and electronic factors heavily favor the ''trans'' isomer in most other peptide bonds. However, peptide bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Transport Protein
A membrane transport protein (or simply transporter) is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral transmembrane proteins; that is they exist permanently within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion or active transport. The two main types of proteins involved in such transport are broadly categorized as either ''channels'' or ''carriers''. The solute carriers and atypical SLCs are secondary active or facilitative transporters in humans. Collectively membrane transporters and channels are known as the transportome. Transportomes govern cellular influx and efflux of not only ions and nutrients but drugs as well. Difference between channels and carriers A carrier is not open simultaneously to both the extracellular and intracellular environments. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimicrobial Peptides
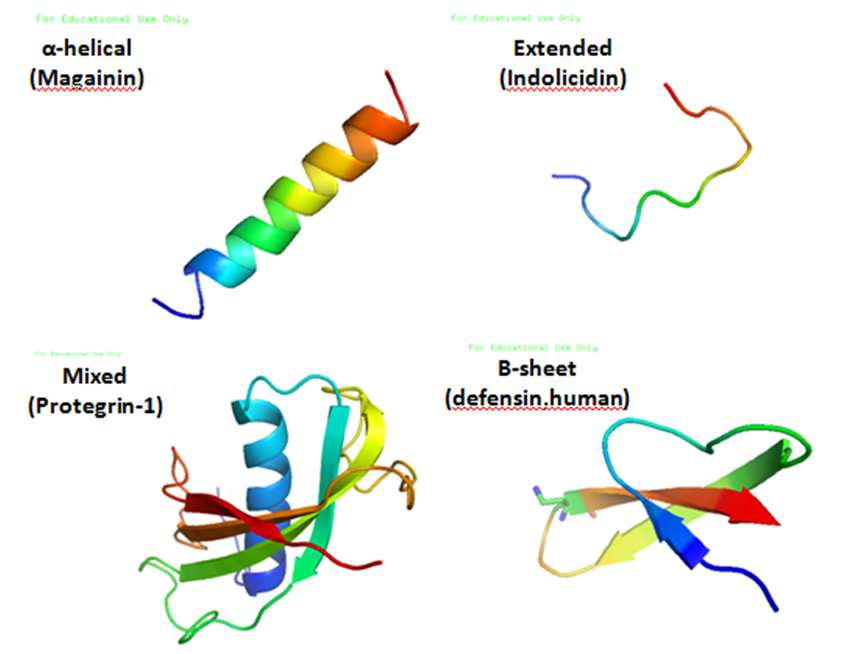
Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antibiotics which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators. Structure Antimicrobial peptides are a unique and diverse group of molecules, which are divided into subgroups on the basis of their amino acid composition and structure. Antimicrobial peptides are g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gramicidin A
Gramicidin, also called gramicidin D, is a mix of ionophoric antibiotics, gramicidin A, B and C, which make up about 80%, 5%, and 15% of the mix, respectively. Each has 2 isoforms, so the mix has 6 different types of gramicidin molecules. They can be extracted from ''Brevibacillus brevis'' soil bacteria. Gramicidins are linear peptides with 15 amino acids. This is in contrast to unrelated gramicidin S, which is a cyclic peptide. Medical uses Gramicidins work as antibiotics against gram-positive bacteria like ''Bacillus subtilis'' and ''Staphylococcus aureus'', but not well against gram-negative ones like ''E. coli''. Gramicidins are used in medicinal lozenges for sore throat and in topical medicines to treat infected wounds. Gramicidins are often mixed with other antibiotics like tyrocidine and antiseptics. Gramicidins are also used in eye drops for bacterial eye infections. In drops, they are often mixed with other antibiotics like polymyxin B or neomycin. Multiple antibiotics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Wall
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are absent in many eukaryotes, including animals, but are present in some other ones like fungi, algae and plants, and in most prokaryotes (except mollicute bacteria). A major function is to act as pressure vessels, preventing over-expansion of the cell when water enters. The composition of cell walls varies between taxonomic group and species and may depend on cell type and developmental stage. The primary cell wall of land plants is composed of the polysaccharides cellulose, hemicelluloses and pectin. Often, other polymers such as lignin, suberin or cutin are anchored to or embedded in plant cell walls. Algae possess cell walls made of glycoproteins and polysaccharides such as carrageenan and agar that are absent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)