|
Dynemicin A
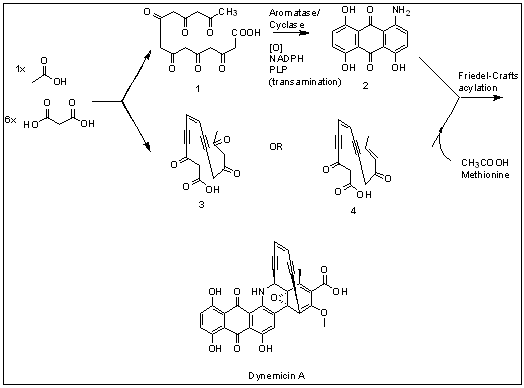
Dynemicin A is an anti-cancer enediyne drug. It displays properties which illustrate promise for cancer treatments, but still requires further research. History and background Dynemicin A was first isolated from the soil in the Gujarat State of India. It was discovered to be the natural product of the indigenous bacteria '' Micromonospora chersina''. The natural product displays a bright purple color due to the anthraquinone chromophore structure within it. Initially, this compound was isolated for its aesthetic properties as a dye until further research demonstrated its anti-cancer properties. Shortly after the compound's discovery, the Bristol-Myers Pharmaceutical Company elucidated the structure in Japan using X-ray diffraction studies of triacetyldynemicin A; a closely related compound. Synthesis The first reported chemical synthesis of dynemicin was accomplished by Myers and coworkers. Biosynthesis Dynemicin A is an antitumor natural product isolated from ''Micro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enediyne
In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond. Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne Chemotherapy, anticancer antibiotics). They are efficient at inducing apoptosis in Cell biology, cells, but cannot differentiate Cancer, cancerous cells from healthy cells. Consequently, research is being conducted to increase the specificity of enediyne toxicity. Structure and reactivity A nine- or ten-membered ring containing a double bond between two triple bonds is termed the warhead of the enediyne. In this state, the warhead is inactive. Enediynes are triggered into a chemically active state via Bergman cyclization, Bergman or Myers-Saito cyclization. The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule. Triggering can also occur via attack by an external nucleophile. Bergman cyclization re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Heterocycles
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bond to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids ( DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes the movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere. Many industrially importa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triols
In chemistry, a triol is a chemical compound containing three hydroxyl groups ( functional groups), such as . See also * , chemical compounds with one hydroxyl group * , chemical compounds with two hydroxyl groups *Polyols
In organic chemistry, a polyol is an organic c ...
[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an ''epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy grou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angucyclines
Angucyclines are antibiotics isolated from ''Streptomyces'' species, which are used in chemotherapy as cytostatics against various types of cancer. The angucyclines include for example aquayamycin, the landomycins, moromycins, saquayamycins, urdamycins, and vineomycins. See also * Anthracyclines Anthracyclines are a class of drugs used in cancer chemotherapy that are extracted from ''Streptomyces'' bacterium. These compounds are used to treat many cancers, including leukemias, lymphomas, breast, stomach, uterine, ovarian, bladder cance ... References {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enediynes
In organic chemistry, enediynes are organic compounds containing two triple bonds and one double bond. Enediynes are most notable for their limited use as antitumor antibiotics (known as enediyne anticancer antibiotics). They are efficient at inducing apoptosis in cells, but cannot differentiate cancerous cells from healthy cells. Consequently, research is being conducted to increase the specificity of enediyne toxicity. Structure and reactivity A nine- or ten-membered ring containing a double bond between two triple bonds is termed the warhead of the enediyne. In this state, the warhead is inactive. Enediynes are triggered into a chemically active state via Bergman or Myers-Saito cyclization. The triggering mechanism can be attributed to an intramolecular nucleophilic attack initiated by one of the variable regions of the molecule. Triggering can also occur via attack by an external nucleophile. Bergman cyclization restructures the enediyne ring into two smaller rings. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cancer Research
Cancer research is research into cancer to identify causes and develop strategies for prevention, diagnosis, treatment, and cure. Cancer research ranges from epidemiology, molecular bioscience to the performance of clinical trials to evaluate and compare applications of the various cancer treatments. These applications include surgery, radiation therapy, chemotherapy, hormone therapy, immunotherapy and combined treatment modalities such as chemo-radiotherapy. Starting in the mid-1990s, the emphasis in clinical cancer research shifted towards therapies derived from biotechnology research, such as cancer immunotherapy and gene therapy. Cancer research is done in academia, research institutes, and corporate environments, and is largely government funded. History Cancer research has been ongoing for centuries. Early research focused on the causes of cancer. Percivall Pott identified the first environmental trigger (chimney soot) for cancer in 1775 and cigarette smoking was identif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neocarzinostatin
Neocarzinostatin (NCS) is a macromolecular chromoprotein enediyne antitumor antibiotic secreted by ''Streptomyces macromomyceticus''. It consists of two parts, a labile chromophore (the non-protein molecular entity shown at right) and a 113 amino acid protein to which the chromophore is tightly and non- covalently bound with high affinity (Kd ~ 10−10 M). The non-protein component is a very potent DNA-damaging agent; However it is extremely unstable and the role of the protein is to protect it and release it to the target DNA. Opening of the epoxide under reductive conditions present in cells creates favorable conditions for a Masamune-Bergman cyclization, leading to formation of benzyne, followed by DNA strand cleavage. Another important member of the chromoprotein group of natural products is kedarcidin. As a medicine it is among the most potent, and in Japan only it has been used against liver cancer clinically. __TOC__ Biosynthesis of NCS Chromophore The biosynthesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leukemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ''leukemia cells''. Symptoms may include bleeding and bruising, bone pain, fatigue, fever, and an increased risk of infections. These symptoms occur due to a lack of normal blood cells. Diagnosis is typically made by blood tests or bone marrow biopsy. The exact cause of leukemia is unknown. A combination of genetic factors and environmental (non-inherited) factors are believed to play a role. Risk factors include smoking, ionizing radiation, petrochemicals (such as benzene), prior chemotherapy, and Down syndrome. People with a family history of leukemia are also at higher risk. There are four main types of leukemia— acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL) and chronic myeloi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Princeton University
Princeton University is a private university, private research university in Princeton, New Jersey. Founded in 1746 in Elizabeth, New Jersey, Elizabeth as the College of New Jersey, Princeton is the List of Colonial Colleges, fourth-oldest institution of higher education in the United States and one of the nine colonial colleges chartered before the American Revolution. It is one of the highest-ranked universities in the world. The institution moved to Newark, New Jersey, Newark in 1747, and then to the current site nine years later. It officially became a university in 1896 and was subsequently renamed Princeton University. It is a member of the Ivy League. The university is governed by the Trustees of Princeton University and has an endowment of $37.7 billion, the largest List of colleges and universities in the United States by endowment, endowment per student in the United States. Princeton provides undergraduate education, undergraduate and graduate education, graduate in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |