|
Drug Research And Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pharmaceutical Drug
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tablet (pharmacy)
A tablet (also known as a pill) is a pharmaceutical oral dosage form (''oral solid dosage'', or OSD) or solid unit dosage form. Tablets may be defined as the solid unit dosage form of medicament or medicaments with suitable excipients. It comprises a mixture of active substances and excipients, usually in powder form, pressed or compacted from a powder into a solid dose. Tablets are prepared either by molding or by compression. The excipients can include diluents, binders or granulating agents, glidants (flow aids) and lubricants to ensure efficient tabletting; disintegrants to promote tablet break-up in the digestive tract; sweeteners or flavours to enhance taste; and pigments to make the tablets visually attractive or aid in visual identification of an unknown tablet. A polymer coating is often applied to make the tablet smoother and easier to swallow, to control the release rate of the active ingredient, to make it more resistant to the environment (extending its shel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capital Expenditure
Capital expenditure or capital expense (capex or CAPEX) is the money an organization or corporate entity spends to buy, maintain, or improve its fixed assets, such as buildings, vehicles, equipment, or land. It is considered a capital expenditure when the asset is newly purchased or when money is used towards extending the useful life of an existing asset, such as repairing the roof. Capital expenditures contrast with operating expenses (opex), which are ongoing expenses that are inherent to the operation of the asset. Opex includes items like electricity or cleaning. The difference between opex and capex may not be immediately obvious for some expenses; for instance, repaving the parking lot may be thought of inherent to the operation of a shopping mall. The dividing line for items like these is that the expense is considered capex if the financial benefit of the expenditure extends beyond the current fiscal year. Usage Capital expenditures are the funds used to acquire or upgra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
New Chemical Entity
A new chemical entity (NCE) is, according to the U.S. Food and Drug Administration, a novel, small, chemical molecule drug that is undergoing clinical trials or has received a first approval (not a new use) by the FDA in any other application submitted under section 505(b) of the Federal Food, Drug, and Cosmetic Act. A new molecular entity (NME) is a broader term that emcompasses both an NCE or an NBE (New Biological Entity). Definition An active moiety is a molecule or ion, excluding those appended portions of the molecule that cause the drug to be an ester, salt (including a salt with hydrogen or coordination bonds), or other noncovalent derivative (such as a complex, chelate, or clathrate) of the molecule, responsible for the physiological or pharmacological action of the drug substance. An NCE is a molecule developed by the innovator company in the early drug discovery stage, which after undergoing clinical trials could translate into a drug that could be a treatment for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pivotal Trial
A pivotal trial is typically a Phase III clinical trial in the multi-year process of clinical research intended to demonstrate and confirm the safety and efficacy of a treatment – such as a drug candidate, medical device or clinical diagnostic procedure – and to estimate the incidence of common adverse effects. A successful pivotal trial is required as evidence for drug marketing approval by the United States Food and Drug Administration (FDA). In drug research, a pivotal Phase III trial may be referred to as a "therapeutic confirmatory study", and is conducted in a large number (hundreds to thousands) of subjects. Such pivotal trials are also designed to discover and estimate the prevalence of common adverse events, but based on their size only have the statistical power to establish an adverse effect rate of not less than 1 in 100 subjects. In an analysis of pivotal trials on medical devices conducted between 2006 and 2013, the median duration was three years, with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Randomized Controlled Trial
A randomized controlled trial (or randomized control trial; RCT) is a form of scientific experiment used to control factors not under direct experimental control. Examples of RCTs are clinical trials that compare the effects of drugs, surgical techniques, medical devices, diagnostic procedures or other medical treatments. Participants who enroll in RCTs differ from one another in known and unknown ways that can influence study outcomes, and yet cannot be directly controlled. By randomly allocating participants among compared treatments, an RCT enables ''statistical control'' over these influences. Provided it is designed well, conducted properly, and enrolls enough participants, an RCT may achieve sufficient control over these confounding factors to deliver a useful comparison of the treatments studied. Definition and examples An RCT in clinical research typically compares a proposed new treatment against an existing standard of care; these are then termed the 'experimental' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Median
In statistics and probability theory, the median is the value separating the higher half from the lower half of a data sample, a population, or a probability distribution. For a data set, it may be thought of as "the middle" value. The basic feature of the median in describing data compared to the mean (often simply described as the "average") is that it is not skewed by a small proportion of extremely large or small values, and therefore provides a better representation of a "typical" value. Median income, for example, may be a better way to suggest what a "typical" income is, because income distribution can be very skewed. The median is of central importance in robust statistics, as it is the most resistant statistic, having a breakdown point of 50%: so long as no more than half the data are contaminated, the median is not an arbitrarily large or small result. Finite data set of numbers The median of a finite list of numbers is the "middle" number, when those numbers are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiology
Cardiology () is a branch of medicine that deals with disorders of the heart and the cardiovascular system. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease and electrophysiology. Physicians who specialize in this field of medicine are called cardiologists, a specialty of internal medicine. Pediatric cardiologists are pediatricians who specialize in cardiology. Physicians who specialize in cardiac surgery are called cardiothoracic surgeons or cardiac surgeons, a specialty of general surgery. Specializations All cardiologists study the disorders of the heart, but the study of adult and child heart disorders each require different training pathways. Therefore, an adult cardiologist (often simply called "cardiologist") is inadequately trained to take care of children, and pediatric cardiologists are not trained to treat adult heart disease. Surgical aspects are not included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
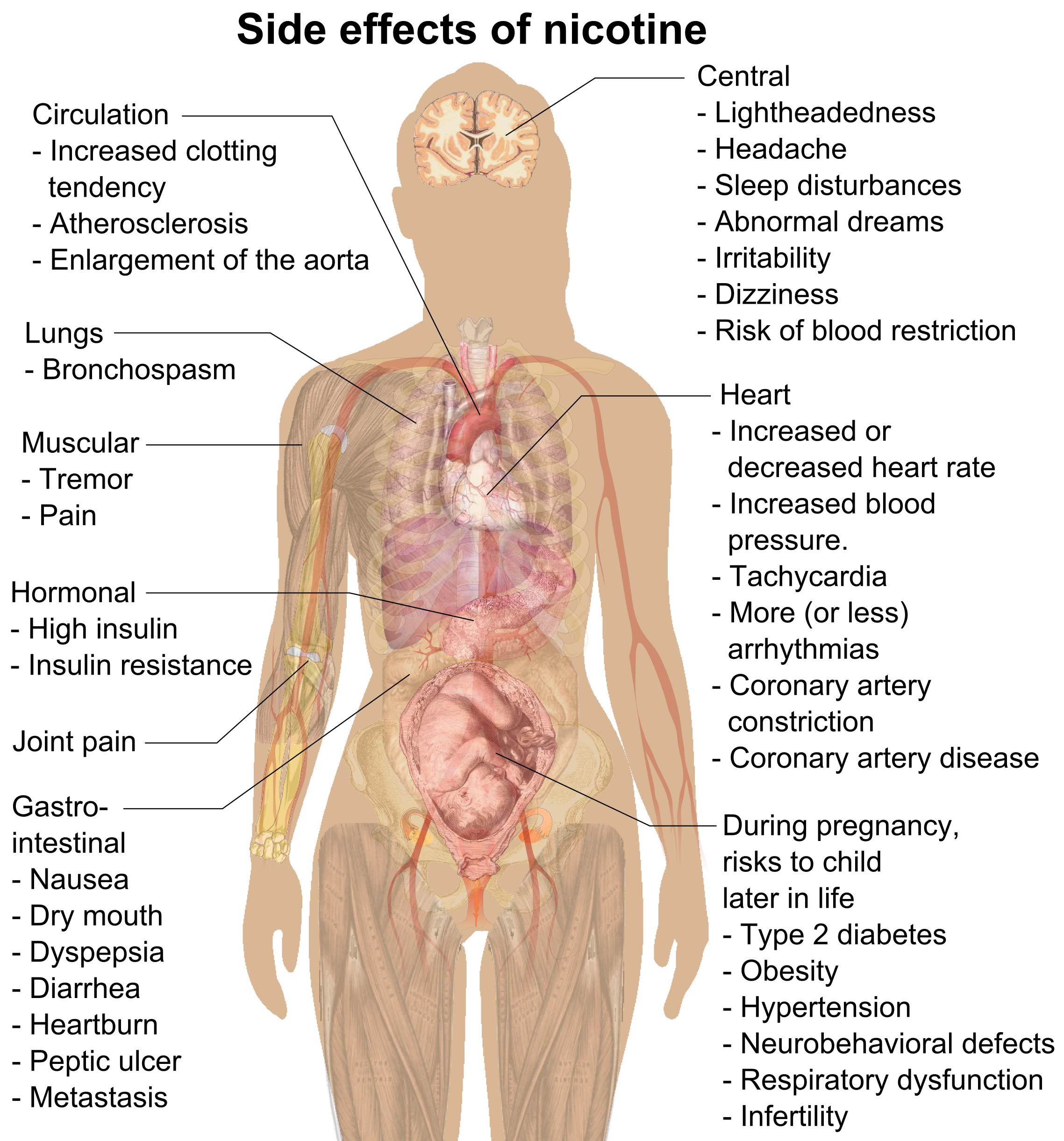
Side Effect
In medicine, a side effect is an effect, whether therapeutic or adverse, that is secondary to the one intended; although the term is predominantly employed to describe adverse effects, it can also apply to beneficial, but unintended, consequences of the use of a drug. Developing drugs is a complicated process, because no two people are exactly the same, so even drugs that have virtually no side effects, might be difficult for some people. Also, it is difficult to make a drug that targets one part of the body but that does not affect other parts, the fact that increases the risk of side effects in the untargeted parts. Occasionally, drugs are prescribed or procedures performed specifically for their side effects; in that case, said side effect ceases to be a side effect and is now an intended effect. For instance, X-rays were historically (and are currently) used as an imaging technique; the discovery of their oncolytic capability led to their employ in radiotherapy (ablation of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Trial
Clinical trials are prospective biomedical or behavioral research studies on human participants designed to answer specific questions about biomedical or behavioral interventions, including new treatments (such as novel vaccines, drugs, dietary choices, dietary supplements, and medical devices) and known interventions that warrant further study and comparison. Clinical trials generate data on dosage, safety and efficacy. They are conducted only after they have received health authority/ethics committee approval in the country where approval of the therapy is sought. These authorities are responsible for vetting the risk/benefit ratio of the trial—their approval does not mean the therapy is 'safe' or effective, only that the trial may be conducted. Depending on product type and development stage, investigators initially enroll volunteers or patients into small pilot studies, and subsequently conduct progressively larger scale comparative studies. Clinical trials can va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)