|
Pivotal Trial
A pivotal trial is typically a Phase III clinical trial in the multi-year process of clinical research intended to demonstrate and confirm the safety and efficacy of a treatment – such as a drug candidate, medical device or clinical diagnostic procedure – and to estimate the incidence of common adverse effects. A successful pivotal trial is required as evidence for drug marketing approval by the United States Food and Drug Administration (FDA). In drug research, a pivotal Phase III trial may be referred to as a "therapeutic confirmatory study", and is conducted in a large number (hundreds to thousands) of subjects. Such pivotal trials are also designed to discover and estimate the prevalence of common adverse events, but based on their size only have the statistical power to establish an adverse effect rate of not less than 1 in 100 subjects. In an analysis of pivotal trials on medical devices conducted between 2006 and 2013, the median duration was three years, with a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phases Of Clinical Research
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Summary Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory authority for use in the general population. Phase IV trials are 'post-marketing' or 'surveillance' studies conducted to monitor safety over sever ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Research
Clinical research is a branch of healthcare science that determines the safety and effectiveness ( efficacy) of medications, devices, diagnostic products and treatment regimens intended for human use. These may be used for prevention, treatment, diagnosis or for relieving symptoms of a disease. Clinical research is different from clinical practice. In clinical practice established treatments are used, while in clinical research evidence is collected to establish a treatment. Overview The term "clinical research" refers to the entire bibliography of a drug/device/biologic, in fact any test article from its inception in the lab to its introduction to the consumer market and beyond. Once the promising candidate or the molecule is identified in the lab, it is subjected to pre-clinical studies or animal studies where different aspects of the test article (including its safety toxicity if applicable and efficacy, if possible at this early stage) are studied. In the United States ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process – from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials – to approved vaccine or drug typically takes more than a decade. New chemical entity development Broadly, the process of drug development can be divided into preclinical and clinical work. Pre-clinical New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds that emerge from the process of drug discovery. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Device
A medical device is any device intended to be used for medical purposes. Significant potential for hazards are inherent when using a device for medical purposes and thus medical devices must be proved safe and effective with reasonable assurance before regulating governments allow marketing of the device in their country. As a general rule, as the associated risk of the device increases the amount of testing required to establish safety and efficacy also increases. Further, as associated risk increases the potential benefit to the patient must also increase. Discovery of what would be considered a medical device by modern standards dates as far back as c. 7000 BC in Baluchistan where Neolithic dentists used flint-tipped drills and bowstrings. Study of archeology and Roman medical literature also indicate that many types of medical devices were in widespread use during the time of ancient Rome. In the United States it wasn't until the Federal Food, Drug, and Cosmetic Act (F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medical Test
A medical test is a medical procedure performed to detect, diagnose, or monitor diseases, disease processes, susceptibility, or to determine a course of treatment. Medical tests such as, physical and visual exams, diagnostic imaging, genetic testing, chemical and cellular analysis, relating to clinical chemistry and molecular diagnostics, are typically performed in a medical setting. Types of tests By purpose Medical tests can be classified by their purposes, the most common of which are diagnosis, screening and evaluation. Diagnostic A diagnostic test is a procedure performed to confirm or determine the presence of disease in an individual suspected of having a disease, usually following the report of symptoms, or based on other medical test results. This includes posthumous diagnosis. Examples of such tests are: * Using nuclear medicine to examine a patient suspected of having a lymphoma. * Measuring the blood sugar in a person suspected of having diabetes mellitus after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adverse Effect
An adverse effect is an undesired harmful effect resulting from a medication or other intervention, such as surgery. An adverse effect may be termed a "side effect", when judged to be secondary to a main or therapeutic effect. The term complication is similar to adverse effect, but the latter is typically used in pharmacological contexts, or when the negative effect is expected or common. If the negative effect results from an unsuitable or incorrect dosage or procedure, this is called a medical error and not an adverse effect. Adverse effects are sometimes referred to as "iatrogenic" because they are generated by a physician/treatment. Some adverse effects occur only when starting, increasing or discontinuing a treatment. Adverse effects can also be caused by placebo treatments (in which case the adverse effects are referred to as nocebo effects). Using a drug or other medical intervention which is contraindicated may increase the risk of adverse effects. Adverse effects may c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drug Approval
An approved drug is a medicinal preparation that has been validated for a therapeutic use by a ruling authority of a government. This process is usually specific by country, unless specified otherwise. Process by country United States In the United States, the FDA approves drugs. Before a drug can be prescribed, it must undergo the FDA's approval process. While a drug can feasibly be used off-label (for non-approved indications), it still is required to be approved for a specific disease or medical condition. Drug companies seeking to sell a drug in the United States must first test it. The company then sends the Food and Drug Administration's Center for Drug Evaluation and Research (CDER) evidence from these tests to prove the drug is safe and effective for its intended use. A fee is required to make such FDA submission. For financial year 2020, this fee was: for an application requiring clinical data ($2,942,965) and for an application not requiring clinical data ($1,471,483 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United States Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a federal agency of the Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, animal foods & feed and veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not directly related to food or drugs, but involves such things as regulating lasers, cellular phones, and condoms, as well as control of disease in contexts varying from h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
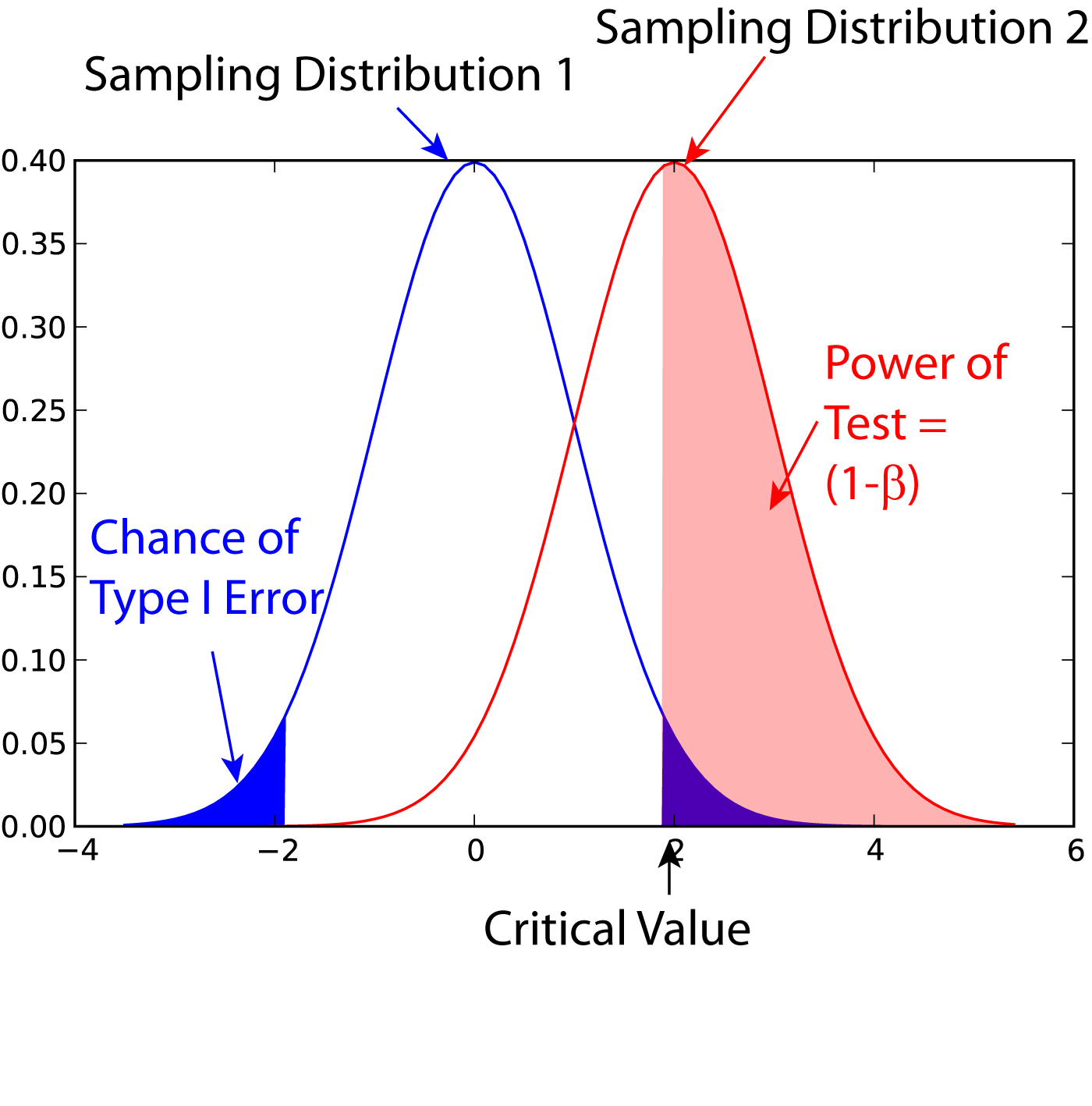
Power (statistics)
In statistics, the power of a binary hypothesis test is the probability that the test correctly rejects the null hypothesis (H_0) when a specific alternative hypothesis (H_1) is true. It is commonly denoted by 1-\beta, and represents the chances of a true positive detection conditional on the actual existence of an effect to detect. Statistical power ranges from 0 to 1, and as the power of a test increases, the probability \beta of making a type II error by wrongly failing to reject the null hypothesis decreases. Notation This article uses the following notation: * ''β'' = probability of a Type II error, known as a "false negative" * 1 − ''β'' = probability of a "true positive", i.e., correctly rejecting the null hypothesis. "1 − ''β''" is also known as the power of the test. * ''α'' = probability of a Type I error, known as a "false positive" * 1 − ''α'' = probability of a "true negative", i.e., correctly not rejecting the null hypothesis Description For a ty ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Median
In statistics and probability theory, the median is the value separating the higher half from the lower half of a data sample, a population, or a probability distribution. For a data set, it may be thought of as "the middle" value. The basic feature of the median in describing data compared to the mean (often simply described as the "average") is that it is not skewed by a small proportion of extremely large or small values, and therefore provides a better representation of a "typical" value. Median income, for example, may be a better way to suggest what a "typical" income is, because income distribution can be very skewed. The median is of central importance in robust statistics, as it is the most resistant statistic, having a breakdown point of 50%: so long as no more than half the data are contaminated, the median is not an arbitrarily large or small result. Finite data set of numbers The median of a finite list of numbers is the "middle" number, when those numbers are list ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clinical Endpoint
Clinical endpoints or clinical outcomes are Outcome measure, outcome measures referring to occurrence of disease, symptom, Medical sign, sign or laboratory abnormality constituting a target outcome in clinical trial, clinical research trials. The term may also refer to any disease or sign that strongly motivates withdrawal of an individual or entity from the trial, then often termed a ''humane (clinical) endpoint''. The primary endpoint of a clinical trial is the endpoint for which the trial is statistical power, powered. Secondary endpoints are additional endpoints, preferably also pre-specified, for which the trial may not be powered. Surrogate endpoint, Surrogate endpoints are trial endpoints that have outcomes that substitute for a clinical endpoint, often because studying the clinical endpoint is difficult, for example using an increase in blood pressure as a surrogate for death by cardiovascular disease, where strong evidence of a causal link exists. Scope In a general sens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surrogate Endpoint
In clinical trials, a surrogate endpoint (or surrogate marker) is a measure of effect of a specific treatment that may correlate with a ''real'' clinical endpoint but does not necessarily have a guaranteed relationship. The National Institutes of Health (USA) defines surrogate endpoint as "a biomarker intended to substitute for a clinical endpoint". Surrogate markers are used when the primary endpoint is undesired (e.g., death), or when the number of events is very small, thus making it impractical to conduct a clinical trial to gather a statistically significant number of endpoints. The FDA and other regulatory agencies will often accept evidence from clinical trials that show a direct clinical benefit to surrogate markers. Surrogate endpoints can be obtained from different modalities, such as, behavioural or cognitive scores, or biomarkers from Electroencephalography ( qEEG), MRI, PET, or biochemical biomarkers. A correlate does not make a surrogate. It is a common misconception ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)