|
Diode Array Detector
A chromatography detector is a device used in gas chromatography (GC) or liquid chromatography (LC) to detect components of the mixture being eluted off the chromatography column. There are two general types of detectors: destructive and non-destructive. The destructive detectors perform continuous transformation of the column effluent (burning, evaporation or mixing with reagents) with subsequent measurement of some physical property of the resulting material (plasma, aerosol or reaction mixture). The non-destructive detectors are directly measuring some property of the column eluent (for example UV absorption) and thus affords greater analyte recovery. Destructive detectors In liquid chromatography: * Charged aerosol detector (CAD) * Evaporative light scattering detector (ELSD) In gas chromatography: * Flame ionization detector (FID) * Flame photometric detector (FPD) * Nitrogen Phosphorus Detector (NPD) * Atomic-emission detector (AED) In all types of chromatography: * Mass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture. In preparative chromatography, GC can be used to prepare pure compounds from a mixture. Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature. Gas chromatography is the process of separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractive Index Detector
A differential refractometer (DRI), or refractive index detector (RI or RID) is a detector that measures the refractive index of an analyte relative to the solvent. They are often used as detectors for high-performance liquid chromatography and size exclusion chromatography. They are considered to be universal detectors because they can detect anything with a refractive index different from the solvent, but they have low sensitivity.Undergraduate Instrumental Methods of Analysis. James W. Robinson, Eileen M. Skelly Frame, George M. Frame II. Marcel Dekker, 2005, p. 810. Principles of operation When light leaves one material and enters another it bends, or refracts. The refractive index of a material is a measure of how much light bends when it enters. Differential refractometers contain a flow cell with two parts: one for the sample and one for the reference solvent. The detector measures the refractive index of both components. When only solvent is passing through the sampl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Chromatography-olfactometry
Gas chromatography-olfactometry (GC-O) is a technique that integrates the separation of volatile compounds using a gas chromatograph with the detection of odour using an olfactometer (human assessor). It was first invented and applied in 1964 by Fuller and co-workers. While GC separates and quantifies volatile compounds from an extract, human olfaction detects the odour activity of each eluting compound. In this olfactometric detection, a human assessor may qualitatively determine whether a compound has odour activity or describe the odour perceived, or quantitatively evaluate the intensity of the odour or the duration of the odour activity. The olfactometric detection of compounds allows the assessment of the relationship between a quantified substance and the human perception of its odour, without instrumental detection limits present in other kinds of detectors. Olfactory perception The properties of a compound relating to human olfactory perception includes its odour quali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photoionization Detector
A photoionization detector or PID is a type of gas detector. Typical photoionization detectors measure volatile organic compounds and other gases in concentrations from sub parts per billion to 10 000 parts per million (ppm). The photoionization detector is an efficient and inexpensive detector for many gas and vapor analytes. PIDs produce instantaneous readings, operate continuously, and are commonly used as detectors for gas chromatography or as hand-held portable instruments. Hand-held, battery-operated versions are widely used in military, industrial, and confined working facilities for health and safety. Their primary use is for monitoring possible worker exposure to volatile organic compounds (VOCs) such as solvents, fuels, degreasers, plastics & their precursors, heat transfer fluids, lubricants, etc. during manufacturing processes and waste handling. Portable PIDs are used for monitoring: * Industrial hygiene and safety * Environmental contamination and remediation * Haza ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
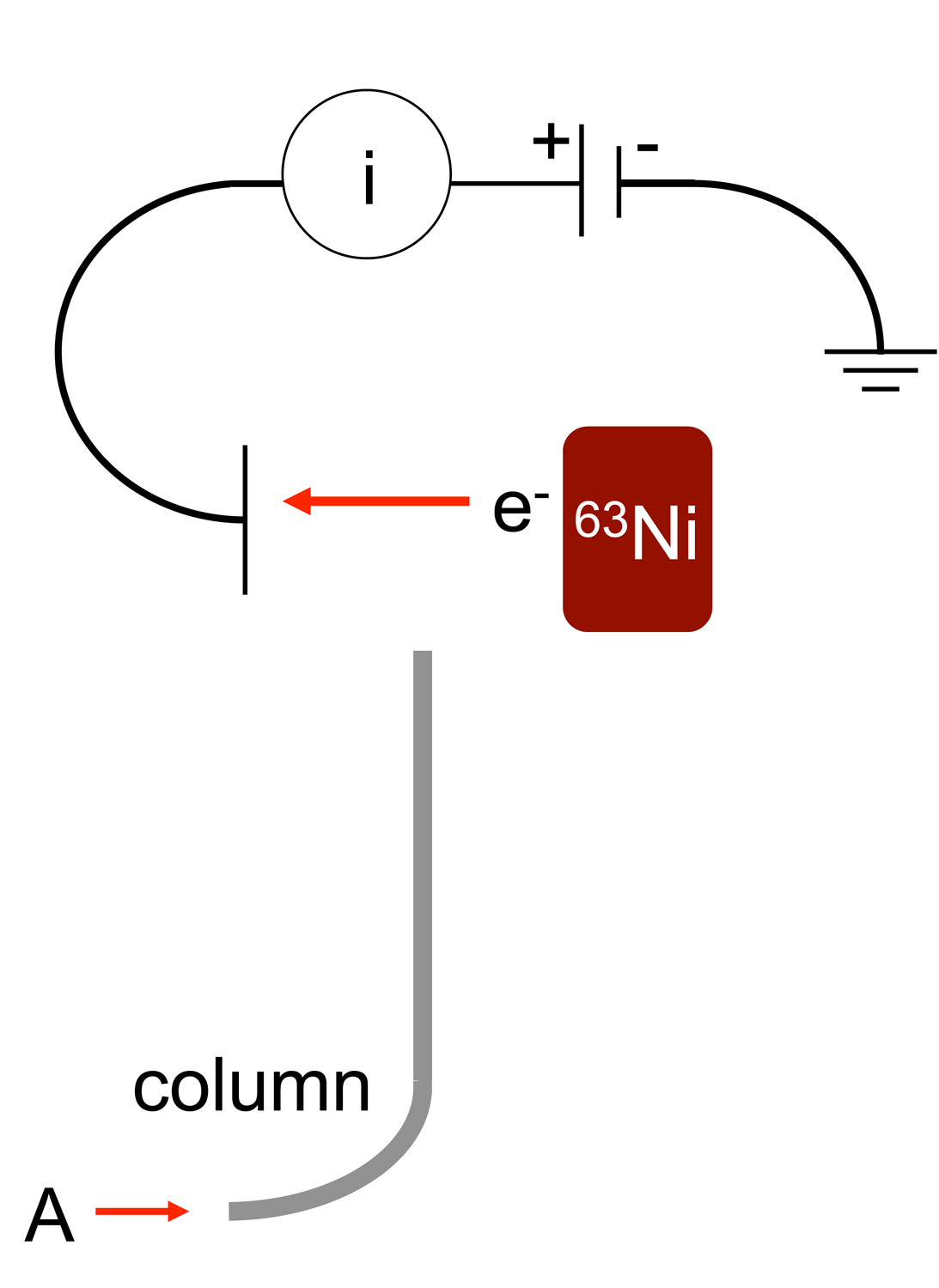
Electron Capture Detector
An electron capture detector (ECD) is a device for detecting atoms and molecules in a gas through the attachment of electrons via electron capture ionization. The device was invented in 1957 by James Lovelock and is used in gas chromatography to detect trace amounts of chemical compounds in a sample. Gas chromatograph detector The electron capture detector is used for detecting electron-absorbing components (high electronegativity) such as halogenated compounds in the output stream of a gas chromatograph. The ECD uses a radioactive beta particle (electron) emitter in conjunction with a so-called makeup gas flowing through the detector chamber. The electron emitter typically consists of a metal foil holding 10 millicuries (370 M Bq) of the radionuclide . Usually, nitrogen is used as makeup gas, because it exhibits a low excitation energy, so it is easy to remove an electron from a nitrogen molecule. The electrons emitted from the electron emitter collide with the molecules of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Conductivity Detector
The thermal conductivity detector (TCD), also known as a katharometer, is a bulk property detector and a chemical specific detector commonly used in gas chromatography. This detector senses changes in the thermal conductivity of the column eluent and compares it to a reference flow of carrier gas. Since most compounds have a thermal conductivity much less than that of the common carrier gases of helium or hydrogen, when an analyte elutes from the column the effluent thermal conductivity is reduced, and a detectable signal is produced. Operation The TCD consists of an electrically heated filament in a temperature-controlled cell. Under normal conditions there is a stable heat flow from the filament to the detector body. When an analyte elutes and the thermal conductivity of the column effluent is reduced, the filament heats up and changes resistance. This resistance change is often sensed by a Wheatstone bridge circuit which produces a measurable voltage change. The column effluent fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Activity
Optical rotation, also known as polarization rotation or circular birefringence, is the rotation of the orientation of the plane of polarization about the optical axis of linearly polarized light as it travels through certain materials. Circular birefringence and circular dichroism are the manifestations of optical activity. Optical activity occurs only in chiral materials, those lacking microscopic mirror symmetry. Unlike other sources of birefringence which alter a beam's state of polarization, optical activity can be observed in fluids. This can include gases or solutions of chiral molecules such as sugars, molecules with helical secondary structure such as some proteins, and also chiral liquid crystals. It can also be observed in chiral solids such as certain crystals with a rotation between adjacent crystal planes (such as quartz) or metamaterials. When looking at the source of light, the rotation of the plane of polarization may be either to the right (dextrorotatory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Scintillation Counting
Liquid scintillation counting is the measurement of radioactive activity of a sample material which uses the technique of mixing the active material with a liquid scintillator (e.g. zinc sulfide), and counting the resultant photon emissions. The purpose is to allow more efficient counting due to the intimate contact of the activity with the scintillator. It is generally used for alpha particle or beta particle detection. Technique Samples are dissolved or suspended in a "cocktail" containing a solvent (historically aromatic organics such as xylene or toluene, but more recently less hazardous solvents are used), typically some form of a surfactant, and "fluors" or scintillators which produce the light measured by the detector. Scintillators can be divided into primary and secondary phosphors, differing in their luminescence properties. Beta particles emitted from the isotopic sample transfer energy to the solvent molecules: the π cloud of the aromatic ring absorbs the energy of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Size Exclusion Chromatography
Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are commonly composed of dextran, agarose, or polyacrylamide polymers. The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers. Applications The main application of gel-filtration chr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence Spectroscopy
Fluorescence spectroscopy (also known as fluorimetry or spectrofluorometry) is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores. Devices that measure fluorescence are called fluorometers. Theory Molecules have various states referred to as energy levels. Fluorescence spectroscopy is primarily concerned with electronic and vibrational states. Generally, the species being examined has a ground electronic state (a low energy state) of interest, and an excited electronic state of higher energy. Within each of these elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system (a column, a capillary tube, a plate, or a sheet) on which a material called the ''stationary phase'' is fixed. Because the different constituents of the mixture tend to have different affinities for the stationary phase and are retained for different lengths of time depending on their interactions with its surface sites, the constituents travel at different apparent velocities in the mobile fluid, causing them to separate. The separation is based on the differential partitioning between the mobile and the stationary phases. Subtle differences in a compound's partition coefficient result in differential retention on the stationary phase and thus affect the separation. Chromatography may be preparative or analytical. The pu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UV Detectors
An ultraviolet detector (also known as UV detector or UV-Vis detector) is a type of non-destructive chromatography detector which measures the amount of ultraviolet or visible light absorbed by components of the mixture being eluted off the chromatography column. They are often used as detectors for high-performance liquid chromatography High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pa .... References {{Chromatography-stub Chromatography ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |