|
Diazotization
Diazonium compounds or diazonium salts are a group of organic compounds sharing a common functional group where R can be any organic group, such as an alkyl or an aryl, and X is an inorganic or organic anion, such as a halide. General properties and reactivity Arenediazonium cations and related species According to X-ray crystallography the linkage is linear in typical diazonium salts. The bond distance in benzenediazonium tetrafluoroborate is 1.083(3) Å, which is almost identical to that for dinitrogen molecule (N≡N). The linear free energy constants σm and σp indicate that the diazonium group is strongly electron-withdrawing. Thus, the diazonio-substituted phenols and benzoic acids have greatly reduced p''K''a values compared to their unsubstituted counterparts. The p''K''a of phenolic proton of 4-hydroxybenzenediazonium is 3.4, versus 9.9 for phenol itself. In other words, the diazonium group lowers the p''K''a (enhances the acidity) by a million-fold. The stabil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Griess
Johann Peter Griess (6 September 1829 – 30 August 1888) was an industrial chemist and an early pioneer of organic chemistry. Griess was influential in the formation of modern dyes, first formulating the diazotization reaction of arylamines. Life After he finished at an agricultural private school, he joined the Hessian cavalry, but left the military shortly after. He started his studies at the University of Jena in 1850, but changed to the University of Marburg in 1851. During his student life he was several times sentenced to the Karzer (campus jail) and was also banned from the city for one year, during which time he listened to lectures of Justus Liebig at the Ludwig Maximilian University of Munich. After most of the family possession had been spent, he had to start working at the chemical factory of Oehler in Offenbach am Main in 1856. This was only possible after the recommendation of Hermann Kolbe, who was head of the chemistry department in Marburg. The devastating fire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Azo Coupling
In organic chemistry, an azo coupling is an organic reaction between a diazonium compound () and another aromatic compound that produces an azo compound (). In this electrophilic aromatic substitution reaction, the aryldiazonium cation is the electrophile and the activated arene is a nucleophile. In most cases, including the examples below, the diazonium compound is also aromatic. Diazotization The process of conversion of primary aromatic amines into its diazonium salt is called diazotization. Diazonium salts are important synthetic intermediates that can undergo coupling reactions to form azo dyes and electrophilic substitution reactions to introduce functional groups. Uses of the reaction Aromatic azo compounds tend to be brightly colored due to the extended conjugated systems. Many are used as dyes (see azo dye). Important azo dyes include methyl red and pigment red 170. Azo printing exploits this reaction as well. Azo coupling is also used to produce prontosil and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzenediazonium Cation
Benzenediazonium tetrafluoroborate is an organic compound with the formula 6H5N2F4. It is a salt of a diazonium cation and tetrafluoroborate. It exists as a colourless solid that is soluble in polar solvents. It is the parent member of the aryldiazonium compounds, which are widely used in organic chemistry. Synthesis Diazotization of aniline in the presence of hydrochloric acid: : C6H5NH2 + HNO2 + HCl → 6H5N2l + 2 H2O The tetrafluoroborate can be obtained from crude benzenediazonium chloride by salt metathesis using tetrafluoroboric acid. : 6H5N2l + HBF4 → 6H5N2F4 + HCl The tetrafluoroborate is more stable than the chloride. Properties The diazo group (N2) can be replaced by many other groups, usually anions, giving a variety of substituted phenyl derivatives: :C6H5N2+ + Nu− → C6H5Nu + N2 These transformations are associated with many named reactions including the Schiemann reaction, Sandmeyer reaction, and Gomberg-Bachmann reaction. A wide range of groups th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on sulfur. This group is usually derived from the compound tosyl chloride, (abbreviated TsCl), which forms esters and amides of toluenesulfonic acid, (abbreviated TsOH). The para orientation illustrated (''p''-toluenesulfonyl) is most common, and by convention ''tosyl'' without a prefix refers to the ''p''-toluenesulfonyl group. The toluenesulfonate (or tosylate) group refers to the – (TsO–) group, with an additional oxygen attached to sulfur and open valence on an oxygen. In a chemical name, the term ''tosylate'' may either refer to the salts containing the anion of ''p''-toluenesulfonic acid, (M = alkali metal, , , etc), or it may refer to esters of ''p''-toluenesulfonic acid, TsOR (R = organyl group). Applications For SN2 reacti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline Yellow
Aniline Yellow is a yellow azo dye and an aromatic amine. It is a derivative of azobenzene. It has the appearance of an orange powder. Aniline Yellow was the first azo dye. it was first produced in 1861 by C. Mene. The second azo dye was Bismarck Brown in 1863. Aniline Yellow was commercialized in 1864 as the first commercial azo dye, a year after Aniline Black. It is manufactured from aniline. Uses Aniline Yellow is used in microscopy for vital staining, in pyrotechnics for yellow colored smokes, in yellow pigments and inks including inks for inkjet printers. It is also used in insecticides, lacquers, varnishes, waxes, oil stains, and styrene resins. It is also an intermediate in synthesis of other dyes, e.g. chrysoidine, indulines, Solid Yellow, and Acid Yellow. Toxic oil syndrome Aniline Yellow was initially implicated in the 1981 Spanish Toxic Oil Syndrome (TOS), although further study suggested the cause was likely contaminated tomatoes. A Madrid-based company imported ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele. Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals. The π electrons do not belong to a single bond or atom, but rather to a group of atoms. Molecules containing conjugated syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
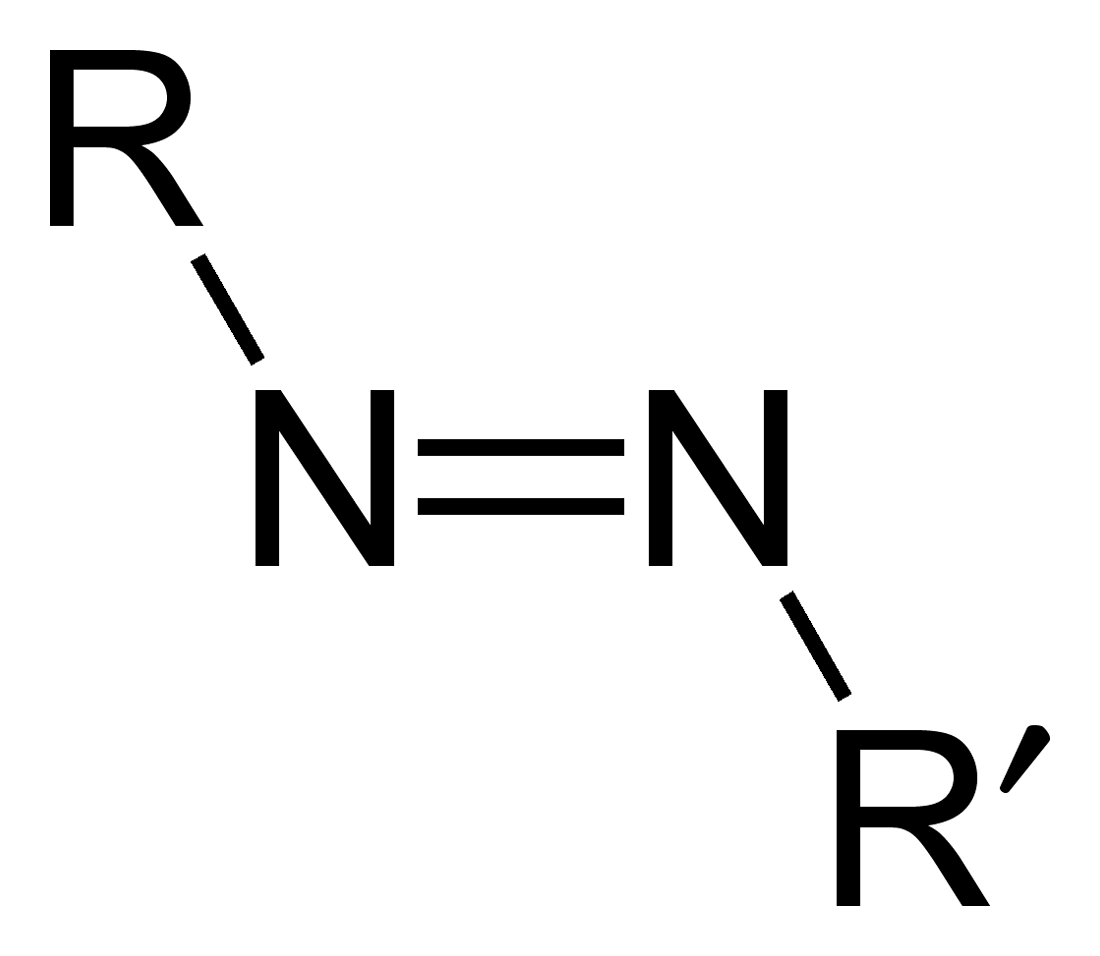
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarylide Pigment
Diarylide pigments are organic compounds that are used as pigments in inks and related materials. They often are yellow or yellow-green. To some extent, these organic compounds have displaced cadmium sulfide from the market. They exist as yellow powders of low solubility in water.K. Hunger. W. Herbst "Pigments, Organic" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2012. Production The formation of these pigments involves the reaction of diazotized aromatic diamines (derivatives of benzidine) with coupling components, typically derivatives of acetoacetanilide. By varying both of these components, a wide variety of pigments have been produced. A related family of organic pigments are the simpler arylides, which arise from the coupling of monodiazonium salts with the same coupling partners. The molecular structures of these molecules is more conjugated than represented in the images shown below. The pigments' colors can range from yellow to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophilic Aromatic Substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic nitration, aromatic halogenation, aromatic sulfonation, and alkylation and acylation Friedel–Crafts reaction. Illustrative reactions The most widely practised example of this reaction is the ethylation of benzene. :: Approximately 24,700,000 tons were produced in 1999. (After dehydrogenation and polymerization, the commodity plastic polystyrene is produced.) In this process, acids are used as catalyst to generate the incipient carbocation. Many other electrophilic reactions of benzene are conducted, although on a much smaller scale; they are valuable routes to key intermediates. The nitration of benzene is achieved via the action of the nitronium ion as the electrophile. The sulfonation with fuming sulfuric acid gives benzenesulfonic ac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |