|
Diammonium Phosphate
Diammonium phosphate (DAP; IUPAC name diammonium hydrogen phosphate; chemical formula (NH4)2(HPO4) is one of a series of water-soluble ammonium phosphate salts that can be produced when ammonia reacts with phosphoric acid. Solid diammonium phosphate shows a dissociation pressure of ammonia as given by the following expression and equation: : At 100 °C, the dissociation pressure of diammonium phosphate is approximately 5 mmHg. According to the diammonium phosphate MSDS from CF Industries, Inc., decomposition starts as low as 70 °C: "Hazardous Decomposition Products: Gradually loses ammonia when exposed to air at room temperature. Decomposes to ammonia and monoammonium phosphate at around 70 °C (158 °F). At 155 °C (311 °F), DAP emits phosphorus oxides, nitrogen oxides and ammonia." Uses DAP is used as a fertilizer. When applied as plant food, it temporarily increases the soil pH, but over a long term the treated ground becomes more acidic than before, upon nitrif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Oxide
Nitrogen oxide may refer to a binary compound of oxygen and nitrogen, or a mixture of such compounds: Charge-neutral *Nitric oxide (NO), nitrogen(II) oxide, or nitrogen monoxide *Nitrogen dioxide (), nitrogen(IV) oxide * Nitrogen trioxide (), or nitrate radical *Nitrous oxide (), nitrogen(0,II) oxide *Dinitrogen dioxide (), nitrogen(II) oxide dimer *Dinitrogen trioxide (), nitrogen(II,IV) oxide *Dinitrogen tetroxide (), nitrogen(IV) oxide dimer *Dinitrogen pentoxide (), nitrogen(V) oxide, or nitronium nitrate *Nitrosyl azide (), nitrogen(−I,0,I,II) oxide * Nitryl azide () *Oxatetrazole () *Trinitramide ( or ), nitrogen(0,IV) oxide Anions *Nitroxide () * Nitrite ( or ) *Nitrate () *Peroxynitrite ( or ) *Peroxynitrate ( or ) *Orthonitrate (, analogous to phosphate ) *Hyponitrite ( or ) *Trioxodinitrate or hyponitrate ( or ) *Nitroxylate ( or ) * Dinitramide ( or ) Cations * Nitrosonium ( or ) * Nitronium ( or ) Atmospheric sciences In atmospheric chemistry: * (or NO''x'') refe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
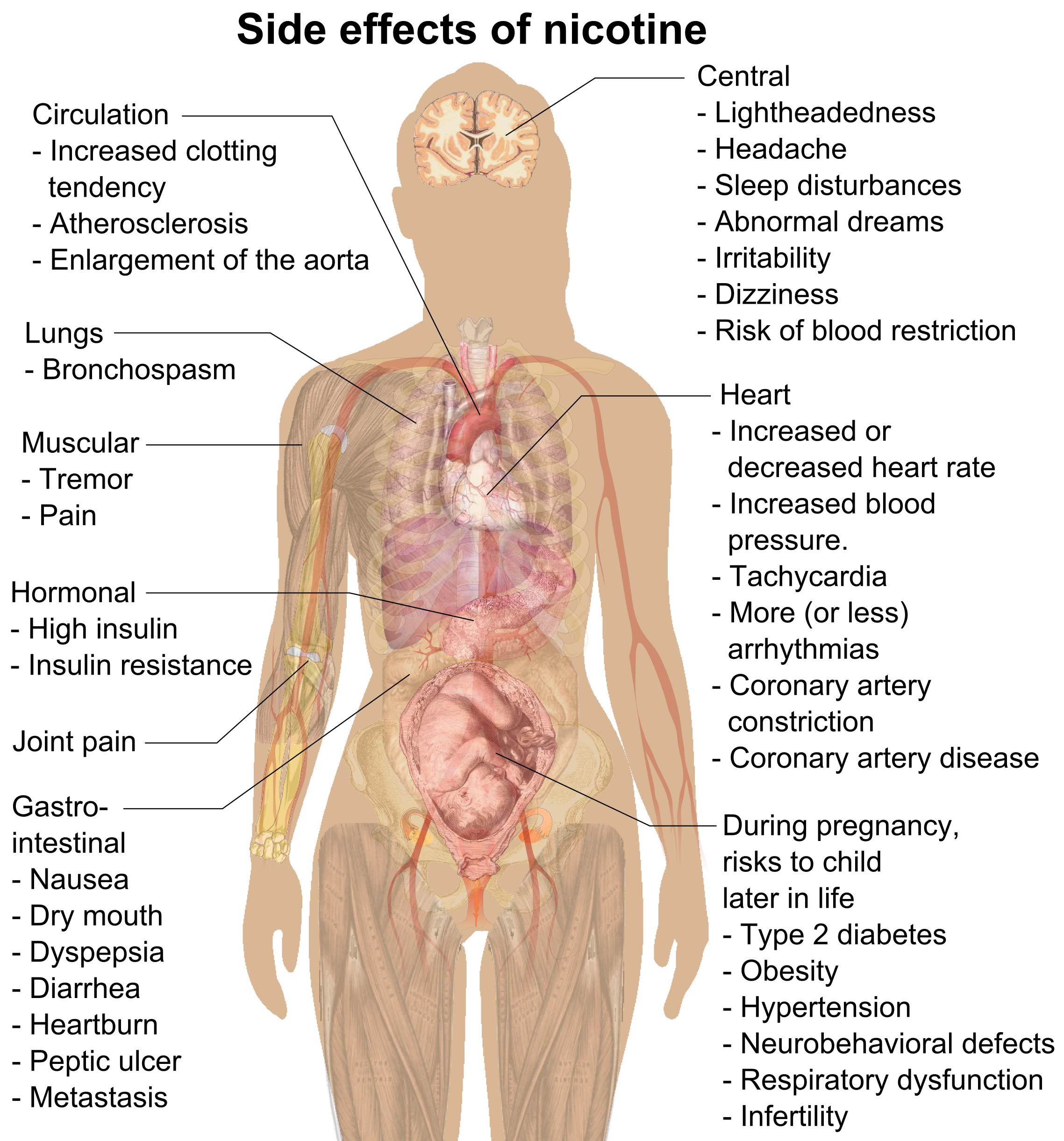
Nicotine
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and ''Duboisia hopwoodii'') and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits (nAChRα9 and nAChRα10) where it acts as a receptor antagonist. Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco. Nicotine is also present at ppb-concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants, though sources disagree on whether this has any biological significance to human consumers. It functions as an antiherbivore toxin; consequently, nicotine was widely used as an insecticide in the past, and neonicotinoids (structurally similar to nicotine), such as imidacloprid, are s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mead
Mead () is an alcoholic beverage made by fermenting honey mixed with water, and sometimes with added ingredients such as fruits, spices, grains, or hops. The alcoholic content ranges from about 3.5% ABV to more than 20%. The defining characteristic of mead is that the majority of the beverage's fermentable sugar is derived from honey. It may be still, carbonated, or naturally sparkling; dry, semi-sweet, or sweet. The term honey wine is sometimes used as a synonym for mead, although ''wine'' is typically defined to be the product of fermented grapes or certain other fruits, and some cultures have honey wines that are distinct from mead. The honey wine of Hungary, for example, is the fermentation of honey-sweetened pomace of grapes or other fruits. Mead was produced in ancient times throughout Europe, Africa, and Asia, and has played an important role in the mythology of some peoples. In Norse mythology, for example, the Mead of Poetry, crafted from the blood of Kvasir (a wise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yeast Assimilable Nitrogen
Yeast assimilable nitrogen or YAN is the combination of free amino nitrogen (FAN), ammonia (NH3) and ammonium (NH4+) that is available for a yeast, e.g. the wine yeast ''Saccharomyces cerevisiae'', to use during fermentation. Outside of the fermentable sugars glucose and fructose, nitrogen is the most important nutrient needed to carry out a successful fermentation that doesn't end prior to the intended point of dryness or sees the development of off-odors and related wine faults. To this extent winemakers will often supplement the available YAN resources with nitrogen additives such as diammonium phosphate (DAP).B. Zoecklein, K. Fugelsang, B. Gump, F. Nury ''Wine Analysis and Production'' pgs 152-163, 340-343, 444-445, 467 Kluwer Academic Publishers, New York (1999) However, the addition of excessive amounts of nitrogen can also create a hazard as other organisms besides beneficial wine yeast can utilize the nutrients. These include spoilage organisms such as ''Brettanomyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Firebreak
A firebreak or double track (also called a fire line, fuel break, fireroad and firetrail in Australia) is a gap in vegetation or other combustible material that acts as a barrier to slow or stop the progress of a bushfire or wildfire. A firebreak may occur naturally where there is a lack of vegetation or "fuel", such as a river, lake or canyon. Firebreaks may also be man-made, and many of these also serve as roads, such as a logging road, four-wheel drive trail, secondary road, or a highway. Overview In the construction of a firebreak, the primary goal is to remove deadwood and undergrowth down to mineral soil. Various methods may be used to accomplish this initially and to maintain this condition. Ideally, the firebreak will be constructed and maintained according to the established practices of sustainable forestry and fire protection engineering, also known as best management practices (BMP). The general goals are to maximize the effectiveness of the firebreak at slowing th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Char (chemistry)
Char is the solid material that remains after light gases (e.g. coal gas) and tar have been driven out or released from a carbonaceous material during the initial stage of combustion, which is known as carbonization, charring, devolatilization or pyrolysis. Further stages of efficient combustion (with or without char deposits) are known as gasification reactions, ending quickly when the reversible gas phase of the water gas shift reaction is reached. See also * Biochar * Charcoal * Coke (fuel) * Petroleum coke * Shale oil extraction * Spent shale Spent shale or spent oil shale (also known as retorted shale) is a solid residue from the shale oil extraction process of producing synthetic shale oil from oil shale. It consists of inorganic compounds (minerals) and remaining organic matter kno ... Oil shale Coal {{Chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and '' lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves , a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |
Wildfires
A wildfire, forest fire, bushfire, wildland fire or rural fire is an unplanned, uncontrolled and unpredictable fire in an area of combustible vegetation. Depending on the type of vegetation present, a wildfire may be more specifically identified as a bushfire( in Australia), desert fire, grass fire, hill fire, peat fire, prairie fire, vegetation fire, or veld fire. Some natural forest ecosystems depend on wildfire. Wildfires are distinct from beneficial human usage of wildland fire, called controlled burning, although controlled burns can turn into wildfires. Fossil charcoal indicates that wildfires began soon after the appearance of terrestrial plants approximately 419 million years ago during the Silurian period. Earth's carbon-rich vegetation, seasonally dry climates, atmospheric oxygen, and widespread lightning and volcanic ignitions create favorable conditions for fires. The occurrence of wildfires throughout the history of terrestrial life invites conjecture that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fire Retardant
A fire retardant is a substance that is used to slow down or stop the spread of fire or reduce its intensity. This is commonly accomplished by chemical reactions that reduce the flammability of fuels or delay their combustion. Fire retardants may also cool the fuel through physical action or endothermic chemical reactions. Fire retardants are available as powder, to be mixed with water, as fire-fighting foams and fire-retardant gels. Fire retardants are also available as coatings or sprays to be applied to an object. Fire retardants are commonly used in fire fighting, where they may be applied aerially or from the ground. Principles of operation In general, fire retardants reduce the flammability of materials by either blocking the fire physically or by initiating a chemical reaction that stops the fire. Physical action There are several ways in which the combustion process can be retarded by physical action: * By cooling: Some chemical reactions actually cool the material d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternary ammonium cations (), where one or more hydrogen atoms are replaced by organic groups (indicated by R). Acid–base properties The ammonium ion is generated when ammonia, a weak base, reacts with Brønsted acids (proton donors): :H+ + NH3 -> H4 The ammonium ion is mildly acidic, reacting with Brønsted bases to return to the uncharged ammonia molecule: : H4 + B- -> HB + NH3 Thus, treatment of concentrated solutions of ammonium salts with strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions: :H2O + NH3 OH- + H4 The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaline
In chemistry, an alkali (; from ar, القلوي, al-qaly, lit=ashes of the saltwort) is a base (chemistry), basic, ionic compound, ionic salt (chemistry), salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline, and less often, alkalescent, is commonly used in English language, English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the acid-base reaction theories#Arrhenius theory, Arrhenius definition of a base, and they are still among the most common bases. Etymology The word "alkali" is derived from Arabic ''al qalīy'' (or ''alkali''), meaning ''the calcined ashes'' (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |