|
Delocalised
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.IUPAC Gold Boo''delocalization''/ref> The term delocalization is general and can have slightly different meanings in different fields: * In organic chemistry, it refers to resonance in conjugated systems and aromatic compounds. * In solid-state physics, it refers to free electrons that facilitate electrical conduction. * In quantum chemistry, it refers to molecular orbital electrons that have extended over several adjacent atoms. Resonance In the simple aromatic ring of benzene, the delocalization of six π electrons over the C6 ring is often graphically indicated by a circle. The fact that the six C-C bonds are equidistant is one indication that the electrons are delocalized; if the structure were to have isolated double bonds alternating with discrete single bonds, the bond would likewise have alternating longer and shorter le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like bond lengths, angles, and dipole moment. Howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metal
A metal (from Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electricity and heat relatively well. Metals are typically ductile (can be drawn into wires) and malleable (they can be hammered into thin sheets). These properties are the result of the ''metallic bond'' between the atoms or molecules of the metal. A metal may be a chemical element such as iron; an alloy such as stainless steel; or a molecular compound such as polymeric sulfur nitride. In physics, a metal is generally regarded as any substance capable of conducting electricity at a temperature of absolute zero. Many elements and compounds that are not normally classified as metals become metallic under high pressures. For example, the nonmetal iodine gradually becomes a metal at a pressure of between 40 and 170 thousand times atmospheric pressure. Equally, some materials regarded as metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. This latter mode forms part of the basis for metal-metal multiple bonding. Pi bonds are usually weaker than sigma bonds. The C-C double bond, composed of one sigma and one pi bond, has a bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromatic Ring Current
An aromatic ring current is an effect observed in aromatic molecules such as benzene and naphthalene. If a magnetic field is directed perpendicular to the plane of the aromatic system, a ring current is induced in the delocalized π electrons of the aromatic ring. This is a direct consequence of Ampère's law; since the electrons involved are free to circulate, rather than being localized in bonds as they would be in most non-aromatic molecules, they respond much more strongly to the magnetic field. The ring current creates its own magnetic field. Outside the ring, this field is in the same direction as the externally applied magnetic field; inside the ring, the field counteracts the externally applied field. As a result, the net magnetic field outside the ring is greater than the externally applied field alone, and is less inside the ring. Aromatic ring currents are relevant to NMR spectroscopy, as they dramatically influence the chemical shifts of 1H nuclei ("protons") in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Unitary Transformation
In mathematics, a unitary transformation is a transformation that preserves the inner product: the inner product of two vectors before the transformation is equal to their inner product after the transformation. Formal definition More precisely, a unitary transformation is an isomorphism between two inner product spaces (such as Hilbert spaces). In other words, a ''unitary transformation'' is a bijective function U : H \to H_2\, between two inner product spaces, H and H_2, such that \langle Ux, Uy \rangle_ = \langle x, y \rangle_ \quad \text x, y \in H. Properties A unitary transformation is an isometry, as one can see by setting x=y in this formula. Unitary operator In the case when H_1 and H_2 are the same space, a unitary transformation is an automorphism of that Hilbert space, and then it is also called a unitary operator. Antiunitary transformation A closely related notion is that of antiunitary transformation, which is a bijective function :U:H_1\to H_2\, between two co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
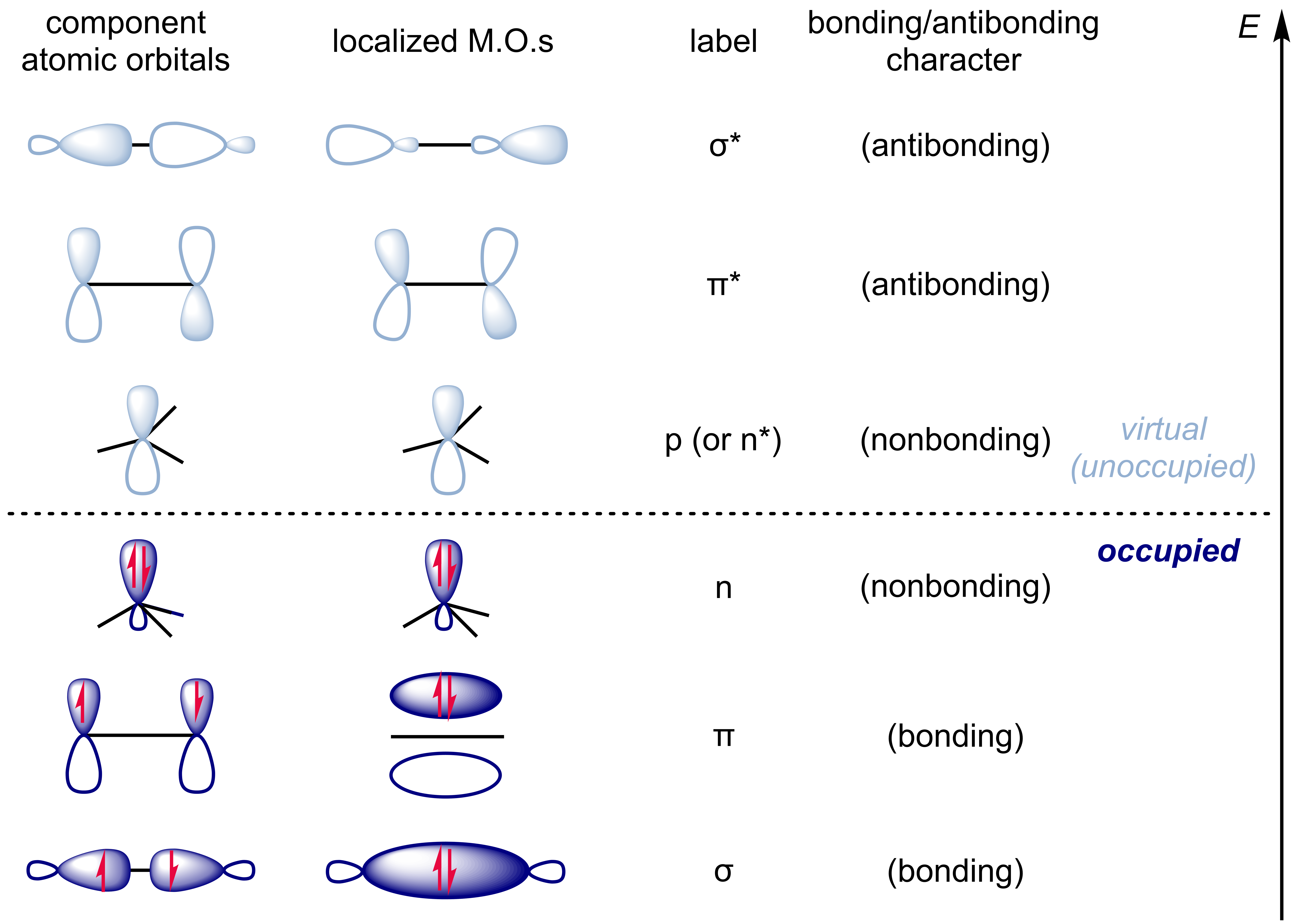
Localized Molecular Orbitals
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The locali ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ab Initio Quantum Chemistry Methods
''Ab initio'' quantum chemistry methods are computational chemistry methods based on quantum chemistry. The term was first used in quantum chemistry by Robert Parr and coworkers, including David Craig in a semiempirical study on the excited states of benzene. The background is described by Parr. ''Ab initio'' means "from first principles" or "from the beginning", implying that the only inputs into an ''ab initio'' calculation are physical constants. ''Ab initio'' quantum chemistry methods attempt to solve the electronic Schrödinger equation given the positions of the nuclei and the number of electrons in order to yield useful information such as electron densities, energies and other properties of the system. The ability to run these calculations has enabled theoretical chemists to solve a range of problems and their importance is highlighted by the awarding of the Nobel prize to John Pople and Walter Kohn. Accuracy and scaling ''Ab initio'' electronic structure method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Right Angle
In geometry and trigonometry, a right angle is an angle of exactly 90 Degree (angle), degrees or radians corresponding to a quarter turn (geometry), turn. If a Line (mathematics)#Ray, ray is placed so that its endpoint is on a line and the adjacent angles are equal, then they are right angles. The term is a calque of Latin ''angulus rectus''; here ''rectus'' means "upright", referring to the vertical perpendicular to a horizontal base line. Closely related and important geometrical concepts are perpendicular lines, meaning lines that form right angles at their point of intersection, and orthogonality, which is the property of forming right angles, usually applied to Euclidean vector, vectors. The presence of a right angle in a triangle is the defining factor for right triangles, making the right angle basic to trigonometry. Etymology The meaning of ''right'' in ''right angle'' possibly refers to the Classical Latin, Latin adjective ''rectus'' 'erect, straight, upright, perp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
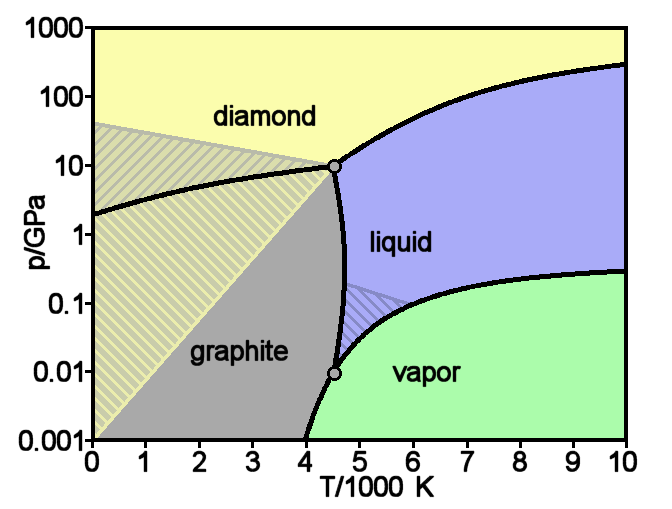
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |