|
Dissipate
In thermodynamics, dissipation is the result of an irreversible process that affects a thermodynamic system. In a dissipative process, energy ( internal, bulk flow kinetic, or system potential) transforms from an initial form to a final form, where the capacity of the final form to do thermodynamic work is less than that of the initial form. For example, transfer of energy as heat is dissipative because it is a transfer of energy other than by thermodynamic work or by transfer of matter, and spreads previously concentrated energy. Following the second law of thermodynamics, in conduction and radiation from one body to another, the entropy varies with temperature (reduces the capacity of the combination of the two bodies to do work), but never decreases in an isolated system. In mechanical engineering, dissipation is the irreversible conversion of mechanical energy into thermal energy with an associated increase in entropy. Processes with defined local temperature produce ent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of thermodynamics, which convey a quantitative description using measurable macroscopic physical quantity, physical quantities but may be explained in terms of microscopic constituents by statistical mechanics. Thermodynamics applies to various topics in science and engineering, especially physical chemistry, biochemistry, chemical engineering, and mechanical engineering, as well as other complex fields such as meteorology. Historically, thermodynamics developed out of a desire to increase the thermodynamic efficiency, efficiency of early steam engines, particularly through the work of French physicist Nicolas Léonard Sadi Carnot, Sadi Carnot (1824) who believed that engine efficiency was the key that could help France win ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Entropy Production
Entropy production (or generation) is the amount of entropy which is produced during heat process to evaluate the efficiency of the process. Short history Entropy is produced in irreversible processes. The importance of avoiding irreversible processes (hence reducing the entropy production) was recognized as early as 1824 by Carnot. In 1865 Rudolf Clausius expanded his previous work from 1854 on the concept of "unkompensierte Verwandlungen" (uncompensated transformations), which, in our modern nomenclature, would be called the entropy production. In the same article in which he introduced the name entropy, Clausius gives the expression for the entropy production for a cyclical process in a closed system, which he denotes by ''N'', in equation (71) which reads :N=S-S_0-\int\frac. Here ''S'' is the entropy in the final state and ''S0'' the entropy in the initial state; ''S0-S'' is the entropy difference for the backwards part of the process. The integral is to be taken from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Computational Physics
Computational physics is the study and implementation of numerical analysis to solve problems in physics. Historically, computational physics was the first application of modern computers in science, and is now a subset of computational science. It is sometimes regarded as a subdiscipline (or offshoot) of theoretical physics, but others consider it an intermediate branch between theoretical and experimental physics — an area of study which supplements both theory and experiment. Overview In physics, different theories based on mathematical models provide very precise predictions on how systems behave. Unfortunately, it is often the case that solving the mathematical model for a particular system in order to produce a useful prediction is not feasible. This can occur, for instance, when the solution does not have a closed-form expression, or is too complicated. In such cases, numerical approximations are required. Computational physics is the subject that deals with these ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also used to describe situations where properties vary systematically, dependent on direction. Isotropic radiation has the same intensity regardless of the direction of measurement, and an isotropic field exerts the same action regardless of how the test particle is oriented. Mathematics Within mathematics, ''isotropy'' has a few different meanings: ; Isotropic manifolds: A manifold is isotropic if the geometry on the manifold is the same regardless of direction. A similar concept is homogeneity. ; Isotropic quadratic form: A quadratic form ''q'' is said to be isotropic if there is a non-zero vector ''v'' such that ; such a ''v'' is an isotropic vector or null vector. In complex geometry, a line through the origin in the direction of an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coherence (physics)
Coherence expresses the potential for two waves to Wave interference, interfere. Two Monochromatic radiation, monochromatic beams from a single source always interfere. Wave sources are not strictly monochromatic: they may be ''partly coherent''. When interfering, two waves add together to create a wave of greater amplitude than either one (constructive Wave interference, interference) or subtract from each other to create a wave of minima which may be zero (destructive interference), depending on their relative phase (waves), phase. Constructive or destructive interference are limit cases, and two waves always interfere, even if the result of the addition is complicated or not remarkable. Two waves with constant relative phase will be coherent. The amount of coherence can readily be measured by the interference visibility, which looks at the size of the interference fringes relative to the input waves (as the phase offset is varied); a precise mathematical definition of the de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hamiltonian Mechanics
In physics, Hamiltonian mechanics is a reformulation of Lagrangian mechanics that emerged in 1833. Introduced by Sir William Rowan Hamilton, Hamiltonian mechanics replaces (generalized) velocities \dot q^i used in Lagrangian mechanics with (generalized) ''momenta''. Both theories provide interpretations of classical mechanics and describe the same physical phenomena. Hamiltonian mechanics has a close relationship with geometry (notably, symplectic geometry and Poisson structures) and serves as a Hamilton–Jacobi equation, link between classical and quantum mechanics. Overview Phase space coordinates (''p'', ''q'') and Hamiltonian ''H'' Let (M, \mathcal L) be a Lagrangian mechanics, mechanical system with configuration space (physics), configuration space M and smooth Lagrangian_mechanics#Lagrangian, Lagrangian \mathcal L. Select a standard coordinate system (\boldsymbol,\boldsymbol) on M. The quantities \textstyle p_i(\boldsymbol,\boldsymbol,t) ~\stackrel~ / are called ''m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Max Planck
Max Karl Ernst Ludwig Planck (; ; 23 April 1858 – 4 October 1947) was a German Theoretical physics, theoretical physicist whose discovery of energy quantum, quanta won him the Nobel Prize in Physics in 1918. Planck made many substantial contributions to theoretical physics, but his fame as a physicist rests primarily on his role as the originator of Quantum mechanics, quantum theory and one of the founders of modern physics, which revolutionized understanding of atomic and Subatomic particle, subatomic processes. He is known for the Planck constant, which is of foundational importance for quantum physics, and which he used to derive a set of Unit of measurement, units, today called Planck units, expressed only in terms of fundamental physical constants. Planck was twice president of the German scientific institution Kaiser Wilhelm Society. In 1948, it was renamed the Max Planck Society (Max-Planck-Gesellschaft) and nowadays includes 83 institutions representing a wide range ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule Heating
Joule heating (also known as resistive heating, resistance heating, or Ohmic heating) is the process by which the passage of an electric current through a conductor (material), conductor produces heat. Joule's first law (also just Joule's law), also known in countries of the former Soviet Union, USSR as the Joule–Lenz law, Assuming the element behaves as a perfect resistor and that the power is completely converted into heat, the formula can be re-written by substituting Ohm's law, V = I R , into the generalized power equation: P = IV = I^2R = V^2/R where ''R'' is the electrical resistance and conductance, resistance. Voltage can be increased in DC circuits by connecting batteries or solar panels in series. Alternating current When current varies, as it does in AC circuits, P(t) = U(t) I(t) where ''t'' is time and ''P'' is the instantaneous active power being converted from electrical energy to heat. Far more often, the ''average'' power is of more interest than the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrical Resistance And Conductance
The electrical resistance of an object is a measure of its opposition to the flow of electric current. Its Multiplicative inverse, reciprocal quantity is , measuring the ease with which an electric current passes. Electrical resistance shares some conceptual parallels with mechanical friction. The International System of Units, SI unit of electrical resistance is the ohm (), while electrical conductance is measured in siemens (unit), siemens (S) (formerly called the 'mho' and then represented by ). The resistance of an object depends in large part on the material it is made of. Objects made of electrical insulators like rubber tend to have very high resistance and low conductance, while objects made of electrical conductors like metals tend to have very low resistance and high conductance. This relationship is quantified by electrical resistivity and conductivity, resistivity or conductivity. The nature of a material is not the only factor in resistance and conductance, howev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Current
An electric current is a flow of charged particles, such as electrons or ions, moving through an electrical conductor or space. It is defined as the net rate of flow of electric charge through a surface. The moving particles are called charge carriers, which may be one of several types of particles, depending on the Electrical conductor, conductor. In electric circuits the charge carriers are often electrons moving through a wire. In semiconductors they can be electrons or Electron hole, holes. In an Electrolyte#Electrochemistry, electrolyte the charge carriers are ions, while in Plasma (physics), plasma, an Ionization, ionized gas, they are ions and electrons. In the International System of Units (SI), electric current is expressed in Unit of measurement, units of ampere (sometimes called an "amp", symbol A), which is equivalent to one coulomb per second. The ampere is an SI base unit and electric current is a ISQ base quantity, base quantity in the International System of Qua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
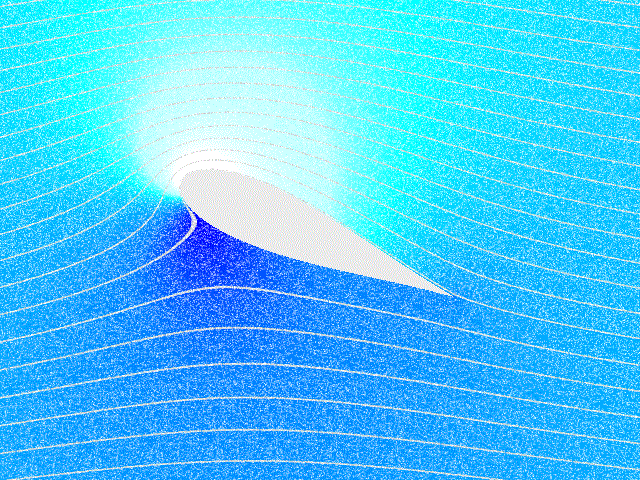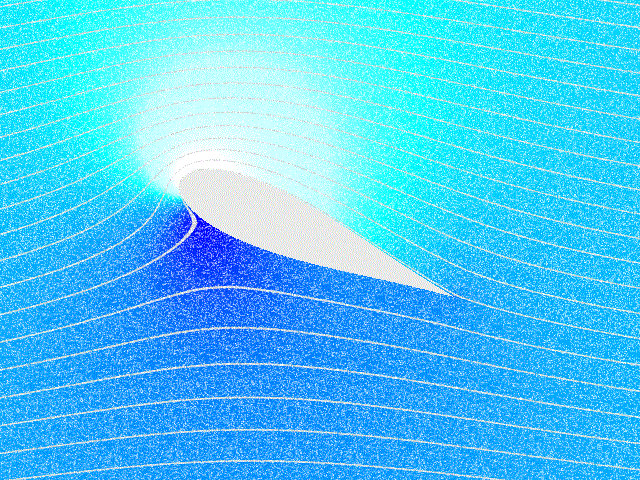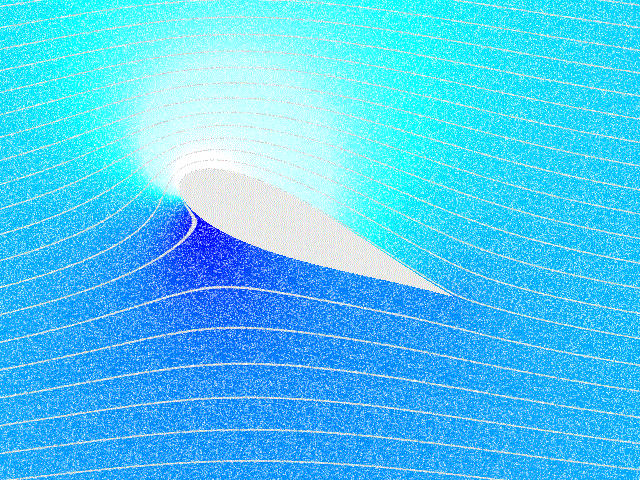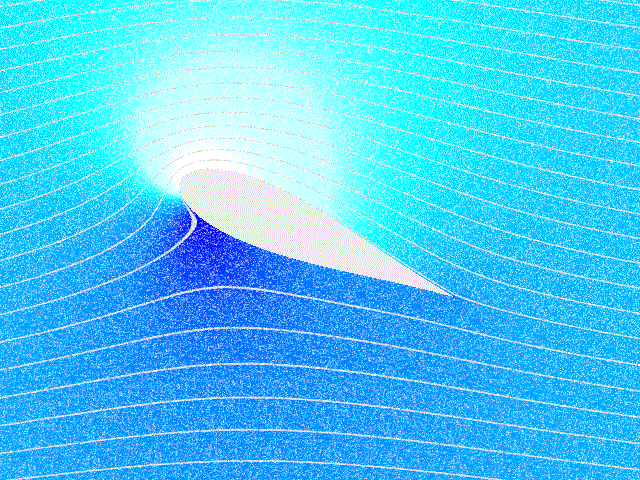
Fluid Flow
In physics, physical chemistry and engineering, fluid dynamics is a subdiscipline of fluid mechanics that describes the flow of fluids – liquids and gases. It has several subdisciplines, including (the study of air and other gases in motion) and (the study of water and other liquids in motion). Fluid dynamics has a wide range of applications, including calculating forces and moment (physics), moments on aircraft, determining the mass flow rate of petroleum through pipeline transport, pipelines, weather forecasting, predicting weather patterns, understanding nebulae in interstellar space, understanding large scale Geophysical fluid dynamics, geophysical flows involving oceans/atmosphere and Nuclear weapon design, modelling fission weapon detonation. Fluid dynamics offers a systematic structure—which underlies these practical disciplines—that embraces empirical and semi-empirical laws derived from flow measurement and used to solve practical problems. The solution to a fl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |