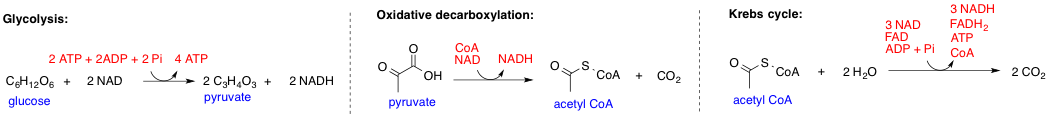|
Dihydroxyacetone Phosphate
Dihydroxyacetone phosphate (DHAP, also glycerone phosphate in older texts) is the anion with the formula HOCH2C(O)CH2OPO32-. This anion is involved in many metabolic pathways, including the Calvin cycle in plants and glycolysis.Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. . It is the phosphate ester of dihydroxyacetone. Role in glycolysis Dihydroxyacetone phosphate lies in the glycolysis metabolic pathway, and is one of the two products of breakdown of fructose 1,6-bisphosphate, along with glyceraldehyde 3-phosphate. It is rapidly and reversibly isomerised to glyceraldehyde 3-phosphate. ''The numbering of the carbon atoms indicates the fate of the carbons according to their position in fructose 6-phosphate.'' Role in other pathways In the Calvin cycle, DHAP is one of the products of the sixfold reduction of 1,3-bisphosphoglycerate by NADPH. It is also used in the synthesis of sedoheptulose 1,7-bisph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolic Pathway
In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell. These enzymes often require dietary minerals, vitamins, and other cofactors to function. Different metabolic pathways function based on the position within a eukaryotic cell and the significance of the pathway in the given compartment of the cell. For instance, the, electron transport chain, and oxidative phosphorylation all take place in the mitochondrial membrane. In contrast, glycolysis, pentose phosphate pathway, and fatty acid biosynthesis all occur in the cytosol of a cell. There are two types of metabolic pathways that are character ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triglycerides
A triglyceride (TG, triacylglycerol, TAG, or triacylglyceride) is an ester derived from glycerol and three fatty acids (from ''tri-'' and ''glyceride''). Triglycerides are the main constituents of body fat in humans and other vertebrates, as well as vegetable fat. They are also present in the blood to enable the bidirectional transference of adipose fat and blood glucose from the liver, and are a major component of human skin oils. Many types of triglycerides exist. One specific classification focuses on saturated and unsaturated types. Saturated fats have ''no'' C=C groups; unsaturated fats feature one or more C=C groups. Unsaturated fats tend to have a lower melting point than saturated analogues; as a result, they are often liquid at room temperature. Chemical structure Triglycerides are tri-esters consisting of a glycerol bound to three fatty acid molecules. Alcohols have a hydroxyl (HO–) group. Organic acids have a carboxyl (–COOH) group. Alcohols and organic a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphates
In organic chemistry, organophosphates (also known as phosphate esters, or OPEs) are a class of organophosphorus compounds with the general structure , a central phosphate molecule with alkyl or aromatic substituents. They can be considered as esters of phosphoric acid. Like most functional groups, organophosphates occur in a diverse range of forms, with important examples including key biomolecules such as DNA, RNA and ATP, as well as many insecticides, herbicides, nerve agents and flame retardants. OPEs have been widely used in various products as flame retardants, plasticizers, and performance additives to engine oil. The popularity of OPEs as flame retardants came as a substitution for the highly regulated brominated flame retardants. The low cost of production and compatibility to diverse polymers made OPEs to be widely used in industry including textile, furniture, electronics as plasticizers and flame retardants. These compounds are added to the final product physi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water – hence the name ''photosynthesis'', from the Greek ''phōs'' (), "light", and ''synthesis'' (), "putting together". Most plants, algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth. Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centers that contain green chlorophyll (and other colored) pigments/chromoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol 3-phosphate Shuttle
The glycerol-3-phosphate shuttle is a mechanism that regenerates NAD+ from NADH, a by-product of glycolysis. The shuttle consists of the sequential activity of two proteins: GPD1 which transfers an electron pair from NADH to dihydroxyacetone phosphate (DHAP), forming glycerol-3-phosphate (G3P) and regenerating NAD+ needed to generate energy via glycolysis. The mitochondrial inner membrane protein GPD2 catalyzes the oxidation of G3P, regenerating DHAP in the cytosol and forming FADH2 in the mitochondrial matrix. In mammals, its activity in transporting reducing equivalents across the mitochondrial membrane is considered secondary to the malate-aspartate shuttle. History The glycerol phosphate shuttle was first characterized as a major route of mitochondrial hydride transport in the flight muscles of blow flies. It was initially believed that the system would be inactive in mammals due to the predominance of lactate dehydrogenase activity over Glycerol-3-phosphate dehydrogenase 1 (G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroxyacetone
Dihydroxyacetone (; DHA), also known as glycerone, is a simple saccharide (a triose) with formula . DHA is primarily used as an ingredient in sunless tanning products. It is often derived from plant sources such as sugar beets and sugar cane, and by the fermentation of glycerin. Chemistry DHA is a hygroscopic white crystalline powder. It has a sweet cooling taste and a characteristic odor. It is the simplest of all ketoses and has no chiral center or optical activity. The normal form is a dimer (2,5-bis(hydroxymethyl)-1,4-dioxane-2,5-diol) which is slowly soluble in one part water and 15 parts ethanol. When freshly prepared, it reverts rapidly to the monomer in solution. : The monomer is very soluble in water, ethanol, diethyl ether, acetone and toluene. DHA may be prepared, along with glyceraldehyde, by the mild oxidation of glycerol, for example with hydrogen peroxide and a ferrous salt as catalyst. It can also be prepared in high yield and selectivity at room temperature fr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Propanediol
Propylene glycol (IUPAC name: propane-1,2-diol) is a viscous, colorless liquid, which is nearly odorless but possesses a faintly sweet taste. Its chemical formula is CH3CH(OH)CH2OH. Containing two alcohol groups, it is classed as a diol. It is miscible with a broad range of solvents, including water, acetone, and chloroform. In general, glycols are non-irritating and have very low volatility. It is produced on a large scale primarily for the production of polymers. In the European Union, it has E-number E1520 for food applications. For cosmetics and pharmacology, the number is E490. Propylene glycol is also present in propylene glycol alginate, which is known as E405. Propylene glycol is a compound which is GRAS (generally recognized as safe) by the US Food and Drug Administration under 21 CFR x184.1666, and is also approved by the FDA for certain uses as an indirect food additive. Propylene glycol is approved and used as a vehicle for topical, oral, and some intravenous pharm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leishmania Mexicana
''Leishmania mexicana'' belongs to the '' Leishmania'' genus and is the causal agent of cutaneous leishmaniasis in Mexico and central America. ''Leishmania mexicana'' is an obligate intracellular protozoan parasite that causes the cutaneous form of leishmaniasis. This species of Leishmania is found in America. The infection with ''L. mexicana'' occurs when an individual is bitten by an infected sandfly that injects infective promastigotes, which are carried in the salivary glands and expulsed by the proboscis, directly to the skin. The life cycle of this and other ''Leishmania'' species begin when an infected Phlebotomine bites and injects promastigotes in the skin of the mammal host. Those promastigotes are engulfed by phagocytic cells, as macrophages and dendritic cells. The parasites are kept inside in a parasitophorous vacuole, then they will transform to amastigotes and divide until brock the cell membrane. They are released to infect new cells as amastigotes stage. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinamide Adenine Dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a coenzyme central to metabolism. Found in all living cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphate groups. One nucleotide contains an adenine nucleobase and the other nicotinamide. NAD exists in two forms: an oxidized and reduced form, abbreviated as NAD and NADH (H for hydrogen), respectively. In metabolism, nicotinamide adenine dinucleotide is involved in redox reactions, carrying electrons from one reaction to another. The cofactor is, therefore, found in two forms in cells: NAD is an oxidizing agent – it accepts electrons from other molecules and becomes reduced. This reaction, also with H+, forms NADH, which can then be used as a reducing agent to donate electrons. These electron transfer reactions are the main function of NAD. However, it is also used in other cellular processes, most notably as a substrate of enzymes in adding or removing chemical groups to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol-3-phosphate Dehydrogenase
''sn''-Glycerol 3-phosphate is the organic ion with the formula HOCH2CH(OH)CH2OPO32-. It is one of three stereoisomers of the ester of dibasic phosphoric acid (HOPO32-) and glycerol. It is a component of glycerophospholipids. Equally appropriate names in biochemical context include glycerol-3-phosphate, 3-''O''-phosphonoglycerol, 3-phosphoglycerol; and Gro3P. From a historical reason, it is also known as -glycerol 3-phosphate, -glycerol 1-phosphate, -α-glycerophosphoric acid. Biosynthesis Glycerol 3-phosphate is synthesized by reducing dihydroxyacetone phosphate (DHAP), an intermediate in glycolysis. The reduction is catalyzed by glycerol-3-phosphate dehydrogenase. DHAP and thus glycerol 3-phosphate can also be synthesized from amino acids and citric acid cycle intermediates via the glyceroneogenesis pathway. : + NAD(P)H + H+ → + NAD(P)+ It is also synthesized by the phosphorylation of glycerol, which is generated by hydrolysis of fats. This esterification is catalyzed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol
Glycerol (), also called glycerine in British English and glycerin in American English, is a simple triol compound. It is a colorless, odorless, viscous liquid that is sweet-tasting and non-toxic. The glycerol backbone is found in lipids known as glycerides. Because it has antimicrobial and antiviral properties, it is widely used in wound and burn treatments approved by the U.S. Food and Drug Administration. Conversely, it is also used as a bacterial culture medium. It can be used as an effective marker to measure liver disease. It is also widely used as a sweetener in the food industry and as a humectant in pharmaceutical formulations. Because of its three hydroxyl groups, glycerol is miscible with water and is hygroscopic in nature. Structure Although achiral, glycerol is prochiral with respect to reactions of one of the two primary alcohols. Thus, in substituted derivatives, the stereospecific numbering labels the molecule with a "sn-" prefix before the stem name of the m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |