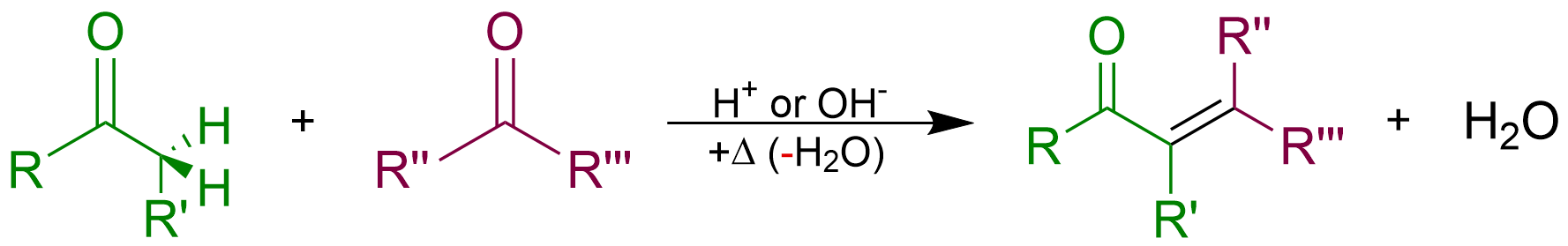|
Dihydrolevoglucosenone
Dihydrolevoglucosenone (Cyrene) is a bicyclic, Chirality (chemistry), chiral, seven-membered heterocyclic cycloalkanone which is a waste derived and fully biodegradable aprotic dipolar solvent. It is an environmentally friendly alternative to dimethylformamide (DMF) and N-Methyl-2-pyrrolidone, N-methyl-2-pyrrolidone (NMP). Preparation Dihydrolevoglucosenone can be prepared through the hydrogenation of unsaturated ketone levoglucosenone (LGO) with Heterogeneous catalysis, heterogenous palladium catalysts under mild conditions. LGO is a chemical building block obtained by acid-catalyzed pyrolysis of lignocellulosic biomass such as sawdust. Properties Dihydrolevoglucosenone is a clear colorless, to light-yellow liquid with a mild, smoky ketone-like odor. It is miscible with water and many organic solvents. Dihydrolevoglucosenone has a boiling point of 226 °C at 101.325 kPa (vs 202 °C for NMP), and a vapor pressure of 14.4 Pa near room temperature (25 °C). I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Levoglucosenone
Levoglucosenone is an organic compound with the formula . A pale yellow liquid, it is an unsaturated bicyclic ketone-diether formed from levoglucosan by loss of two molecules of water. As a product of the acid-catalysed pyrolysis of cellulose, Glucose, D-glucose, and levoglucosan, this liquid hydrocarbon is of interest as a biofuel and biofeedstock. Production The compound was first identified in 1970 as a product of the thermal decomposition of cellulose. The primary way of obtaining levoglucosenone is via pyrolysis of Carbohydrate, carbohydrates, particularly cellulose. Levoglucosenone can be derived from biomass or from other cellulosic materials including domestic/commercial waste paper. The availability of multiple sources is a key advantage when compared to other platform chemicals which are solely derived from biomass. The title compound is produced when cellulose is heated above 170 °C with sulfuric acid with various additives. Alongside levoglucosenone as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic
In chemistry, a bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spirocyclic compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the so-called bridgehead atoms are directly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexamethylenediamine
Hexamethylenediamine is the organic compound with the formula H2N(CH2)6NH2. The molecule is a diamine, consisting of a hexamethylene hydrocarbon chain terminated with amine functional groups. The colorless solid (yellowish for some commercial samples) has a strong amine odor. About 1 billion kilograms are produced annually.Robert A. Smiley "Hexamethylenediamine" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. Synthesis Hexamethylenediamine was first reported by Theodor Curtius. It is produced by the hydrogenation of adiponitrile: :NC(CH2)4CN + 4 H2 → H2N(CH2)6NH2 The hydrogenation is conducted on molten adiponitrile diluted with ammonia, typical catalysts being based on cobalt and iron. The yield is good, but commercially significant side products are generated by virtue of reactivity of partially hydrogenated intermediates. These other products include 1,2-diaminocyclohexane, hexamethyleneimine, and the triamine bis(hexamethylenetriam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyurethane
Polyurethane (; often abbreviated PUR and PU) refers to a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane is produced from a wide range of starting materials. This chemical variety produces polyurethanes with different chemical structures leading to many List of polyurethane applications, different applications. These include rigid and flexible foams, varnishes and coatings, adhesives, Potting (electronics), electrical potting compounds, and fibers such as spandex and Polyurethane laminate, PUL. Foams are the largest application accounting for 67% of all polyurethane produced in 2016. A polyurethane is typically produced by reacting an isocyanate with a polyol. Since a polyurethane contains two types of monomers, which polymerize one after the other, they are classed as Copolymer#Alternating copolymers, alternating copolymers. Both the isocy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyester
Polyester is a category of polymers that contain the ester functional group in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include naturally occurring chemicals, such as in plants and insects, as well as synthetics such as polybutyrate. Natural polyesters and a few synthetic ones are biodegradable, but most synthetic polyesters are not. Synthetic polyesters are used extensively in clothing. Polyester fibers are sometimes spun together with natural fibers to produce a cloth with blended properties. Cotton-polyester blends can be strong, wrinkle- and tear-resistant, and reduce shrinking. Synthetic fibers using polyester have high water, wind and environmental resistance compared to plant-derived fibers. They are less Fireproofing, fire-resistant and can melt when ignited. Liquid crystalline polyesters are among the first industrially used liquid crystal polymers. They are use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,6-Hexanediol
1,6-Hexanediol is an organic compound with the formula (CH2CH2CH2OH)2. It is a colorless water-soluble solid. Production 1,6-Hexanediol is prepared by the hydrogenation of adipic acid or its esters. Laboratory preparation could be achieved by reduction of adipates with lithium aluminium hydride, although this method is impractical on a commercial scale. Properties As 1,6-hexanediol contains the hydroxyl group, it undergoes the typical chemical reactions of alcohols such as dehydration, substitution, esterification. Dehydration of 1,6-hexanediol gives oxepane, 2-methyltetrahydropyran and 2-ethyltetrahydrofuran. Corresponding thiophene and pyrrolidone can be made by reacting 1,6-hexanediol with hydrogen sulfide and ammonia respectively.BASF intermediates, BASF Uses 1,6-Hexanediol is widely used for industrial polyester and polyurethane production.. 1,6-Hexanediol can improve the hardness and flexibility of polyesters as it contains a fairly long hydrocarbon chain. In polyuret ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation Von Dihydrolevoglucosenon
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zalcitabine
Zalcitabine (2′-3′-dideoxycytidine, ddC), also called dideoxycytidine, is a nucleoside analog reverse-transcriptase inhibitor (NRTI) sold under the trade name Hivid. Zalcitabine was the third antiretroviral to be approved by the Food and Drug Administration (FDA) for the treatment of HIV/AIDS. It is used as part of a combination regimen. Zalcitabine appears less potent than some other nucleoside RTIs, has an inconvenient three-times daily frequency and is associated with serious adverse events. For these reasons it is now rarely used to treat human immunodeficiency virus (HIV), and it has even been removed from pharmacies entirely in some countries. History Zalcitabine was first synthesized in the 1960s by Jerome HorwitzOral account of the history of AZT, d4T and ddC by Jerome Horwitz and Hiroaki Mitsuya in the documentary filI am alive today - History of an AIDS drug and subsequently developed as an anti-HIV agent by Samuel Broder, Hiroaki Mitsuya, and Robert Yarchoan at the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peracetic Acid
Peracetic acid (also known as peroxyacetic acid, or PAA) is an organic compound with the formula CH3CO3H. This peroxy acid is a colorless liquid with a characteristic acrid odor reminiscent of acetic acid. It can be highly corrosive. Peracetic acid is a weaker acid than the parent acetic acid, with a p''K''a of 8.2. Production Peracetic acid is produced industrially by the autoxidation of acetaldehyde: :O2 + CH3CHO → CH3CO3H It forms upon treatment of acetic acid with hydrogen peroxide with a strong acid catalyst: :H2O2 + CH3CO2H CH3CO3H + H2O As an alternative, acetyl chloride and acetic anhydride can be used to generate a solution of the acid with lower water content. Peracetic acid is generated ''in situ'' by some laundry detergents. This is achieved by the action of bleach activators, such as tetraacetylethylenediamine and sodium nonanoyloxybenzenesulfonate, upon hydrogen peroxide formed from sodium percarbonate in water. The peracetic acid is a more effective bleachi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxy Acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy derivatives of organic carboxylic acids. They are generally strong oxidizers. Inorganic peroxy acids Peroxymonosulfuric acid (Caro's acid) is probably the most important inorganic peracid, at least in terms of its production scale. It is used for the bleaching of pulp and for the detoxification of cyanide in the mining industry. It is produced by treating sulfuric acid with hydrogen peroxide. Peroxymonophosphoric acid (H3PO5) is prepared similarly. Organic peracids Several organic peroxyacids are commercially useful. They can be prepared in several ways. Most commonly, peracids are generated by treating the corresponding carboxylic acid with hydrogen peroxide: :RCO2H + H2O2 RCO3H + H2O A related reaction involves treatment o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chiral Pool
The chiral pool is a "collection of abundant enantiopure building blocks provided by nature" used in synthesis. In other words, a chiral pool would be a large quantity of common organic enantiomers. Contributors to the chiral pool are amino acids, sugars, and terpenes. Their use improves the efficiency of total synthesis. Not only does the chiral pool contribute a premade carbon skeleton, their chirality is usually preserved in the remainder of the reaction sequence. This strategy is especially helpful if the desired molecule resembles cheap enantiopure natural products. Many times, suitable enantiopure starting materials cannot be identified. The alternative to the use of the chiral pool is asymmetric synthesis, whereby achiral precursors are employed or racemic intermediates are resolved. Examples The use of the chiral pool is illustrated by the synthesis of the anticancer drug paclitaxel (Taxol). The incorporation of the C10 precursor verbenone, a member of the chiral pool, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |