|
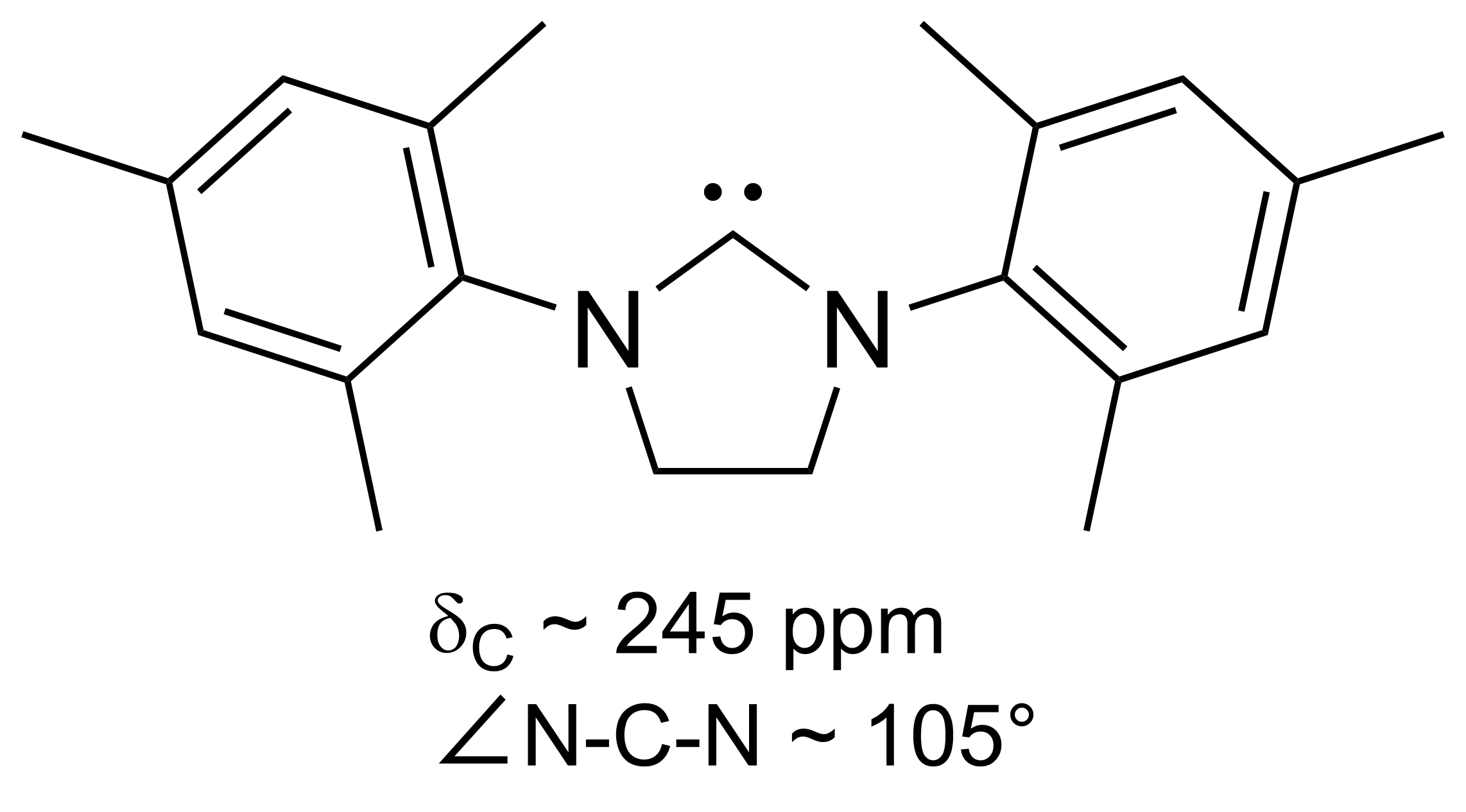
Diaminocarbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be isol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SIMes
SIMes (or H2Imes) is an ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry. It is structurally related to the more common ligand IMes but with a saturated backbone (the S of SIMes indicates a saturated backbone). It is slightly more flexible and is a component in Grubbs II. It is prepared by alkylation of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation. References {{organic-compound-stub Carbenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vitamin B1
Thiamine, also known as thiamin and vitamin B1, is a vitamin, an essential micronutrient, that cannot be made in the body. It is found in food and commercially synthesized to be a dietary supplement or medication. Phosphorylated forms of thiamine are required for some metabolic reactions, including the breakdown of glucose and amino acids. Food sources of thiamine include whole grains, legumes, and some meats and fish. Grain processing removes much of the vitamin content, so in many countries cereals and flours are enriched with thiamine. Supplements and medications are available to treat and prevent thiamine deficiency and disorders that result from it include beriberi and Wernicke encephalopathy. They are also used to treat maple syrup urine disease and Leigh syndrome. Supplements and medications are typically taken by mouth, but may also be given by intravenous or intramuscular injection. Thiamine supplements are generally well tolerated. Allergic reactions, including a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''pyro'' "fire", "heat", "fever" and '' lysis'' "separating". Pyrolysis is most commonly used in the treatment of organic materials. It is one of the processes involved in charring wood.''Burning of wood'' , InnoFireWood's website. Accessed on 2010-02-06. In general, pyrolysis of organic substances produces volatile products and leaves char, a carbon-rich solid residue. Extreme pyrolysis, which leaves mostly |
Dihydroimidazol-2-ylidene
Dihydroimidazol-2-ylidene is a hypothetical organic compound with formula C3H6N2. It would be a heterocyclic compound, formally derived from imidazolidine with two hydrogen atoms removed from carbon number 2, leaving two vacant chemical bonds — which makes it a carbene. Although carbenes in general are extremely short-lived, some derivatives of this compound are surprisingly stable, and form an important class of the persistent carbenes. They include the first stable carbenes postulated (but not isolated) by Hans-Werner Wanzlick around 1960. They also include an example of the (saturated) imidazolin-2-ylidene (carbene In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms. The term "carbene" ma ...) reported by A.J. Arduengo in 1995. References Carbenes Imidazolines Hypothetical chemical compound ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hans-Werner Wanzlick
Hans-Werner Wanzlick (1917-1988) was a German chemist. A Professor of chemistry at the Berlin Technical University he is notable for work on persistent carbenes and for proposing the Wanzlick equilibrium between saturated imidazolin-2-ylidenes and their dimer Dimer may refer to: * Dimer (chemistry), a chemical structure formed from two similar sub-units ** Protein dimer, a protein quaternary structure ** d-dimer * Dimer model, an item in statistical mechanics, based on ''domino tiling'' * Julius Dimer ...s — which he called "''das doppelte Lottchen''", after a 1949 novel by Erich Kästner about a pair of mischievous twins. Anthony J. Arduengo, III (1999) ''Looking for Stable Carbenes: The Difficulty in Starting Anew''. Accounts of Chemical Research, volume 32, number 11, pages 913–921. References * Hans-Werner Wanzlick (1958), letter to Linus Pauling (in German). Linus Pauling's Correspondence, item #444.6. Technical University of Berlin faculty 19 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deuteron
Deuterium (or hydrogen-2, symbol or deuterium, also known as heavy hydrogen) is one of two stable isotopes of hydrogen (the other being protium, or hydrogen-1). The nucleus of a deuterium atom, called a deuteron, contains one proton and one neutron, whereas the far more common protium has no neutrons in the nucleus. Deuterium has a natural abundance in Earth's oceans of about one atom of deuterium among all atoms of hydrogen (see heavy water). Thus deuterium accounts for approximately 0.0156% by number (0.0312% by mass) of all the naturally occurring hydrogen in the oceans, while protium accounts for more than 99.98%. The abundance of deuterium changes slightly from one kind of natural water to another (see Vienna Standard Mean Ocean Water). (Tritium is yet another hydrogen isotope, with two neutrons, that is far more rare and is radioactive.) The name ''deuterium'' is derived from the Greek , meaning "second", to denote the two particles composing the nucleus. Deuteri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ratio). Protons and neutrons, each with masses of approximately one atomic mass unit, are jointly referred to as "nucleons" (particles present in atomic nuclei). One or more protons are present in the nucleus of every atom. They provide the attractive electrostatic central force which binds the atomic electrons. The number of protons in the nucleus is the defining property of an element, and is referred to as the atomic number (represented by the symbol ''Z''). Since each element has a unique number of protons, each element has its own unique atomic number, which determines the number of atomic electrons and consequently the chemical characteristics of the element. The word ''proton'' is Greek for "first", and this name was given to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazolium
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearly indicating ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |