|
Decarboxylative
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases ( EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the corresponding aryl anion, which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kolbe Electrolysis
__NOTOC__ The Kolbe electrolysis or Kolbe reaction is an organic reaction named after Hermann Kolbe. The Kolbe reaction is formally a decarboxylative dimerisation of two carboxylic acids (or carboxylate ions). The overall reaction is: : If a mixture of two different carboxylates are used, all combinations of them are generally seen as the organic product structures: : 3 R1COO− + 3 R2COO− → R1−R1 + R1−R2 + R2−R2 + 6 CO2 + 6 e− The reaction mechanism involves a two-stage radical process: electrochemical decarboxylation gives a radical intermediate, which combine to form a covalent bond. As an example, electrolysis of acetic acid yields ethane and carbon dioxide: :CH3COOH → CH3COO− → CH3COO· → CH3· + CO2 :2CH3· → CH3CH3 Another example is the synthesis of 2,7-dimethyl-2,7-dinitrooctane from 4-methyl-4-nitrovaleric acid: : Further reading * * See also * Electrosynthesis Electrosynthesis in chemistry is the synthesis of chemical compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
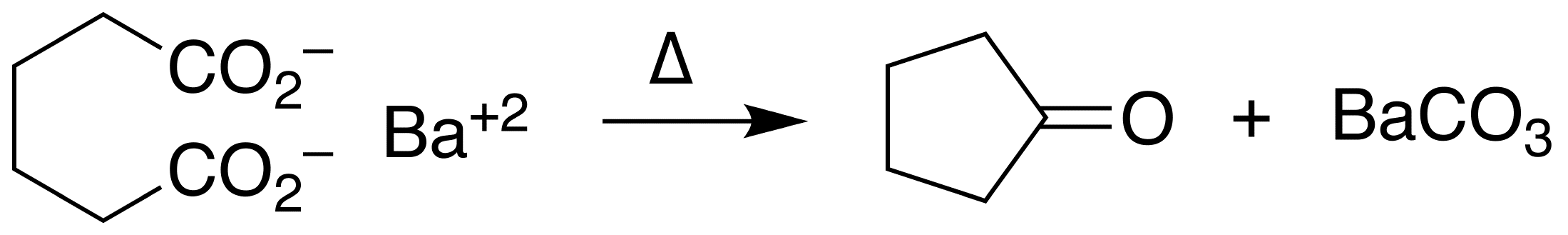
Ketonic Decarboxylation
In organic chemistry, ketonic decarboxylation (also known as decarboxylative ketonization) is a type of organic reaction and a decarboxylation converting two equivalents of a carboxylic acid () to a symmetric ketone () by the application of heat with expulsion of one equivalent of water () and one equivalent of carbon dioxide (): :\ce\mathbf + \ce\mathbf \longrightarrow \ce\mathbf + \ce Bases promote this reaction. The reaction mechanism likely involves nucleophilic attack of the alpha-carbon of one acid group on the other acid group's carbonyl (), possibly as a concerted reaction with the decarboxylation. The initial formation of an intermediate carbanion via decarboxylation of one of the acid groups prior to the nucleophilic attack has been proposed, but is unlikely since the byproduct resulting from the carbanion's protonation by the acid has never been reported. This reaction is different from oxidative decarboxylation, which proceeds through a radical mechanism and is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxyl Group
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barton Decarboxylation
The Barton decarboxylation is a radical reaction in which a carboxylic acid is converted to a thiohydroxamate ester (commonly referred to as a Barton ester). The product is then heated in the presence of a radical initiator and a suitable hydrogen donor to afford the decarboxylated product. This is an example of a reductive decarboxylation. Using this reaction it is possible to remove carboxylic acid moieties from alkyl groups and replace them with other functional groups. (See Scheme 1) This reaction is named after its developer, the British chemist and Nobel laureate Sir Derek Barton (1918–1998). : Mechanism The reaction is initiated by homolytic cleavage of a radical initiator, in this case 2,2'-azobisisobutyronitrile ( AIBN), upon heating. A hydrogen is then abstracted from the hydrogen source ( tributylstannane in this case) to leave a tributylstannyl radical that attacks the sulfur atom of the thiohydroxamate ester. The N-O bond of the thiohydroxamate ester undergoes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kochi Reaction
The Kochi reaction is an organic reaction for the decarboxylation of carboxylic acid In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...s to alkyl halides with lead(IV) acetate and a lithium halide.''A New Method for Halodecarboxylation of Acids Using Lead(IV) Acetate'' Jay K. Kochi J. Am. Chem. Soc.; 1965; 87(11); 2500–02. The reaction is a variation of the Hunsdiecker reaction. References Organic reactions Name reactions {{organic-chemistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hunsdiecker Reaction
The Hunsdiecker reaction (also called the Borodin reaction or the Hunsdiecker–Borodin reaction) is a name reaction in organic chemistry whereby silver salts of carboxylic acids react with a halogen to produce an organic halide. It is an example of both a decarboxylation and a halogenation reaction as the product has one fewer carbon atoms than the starting material (lost as carbon dioxide) and a halogen atom is introduced its place. The reaction was first demonstrated by Alexander Borodin in his 1861 reports of the preparation of methyl bromide () from silver acetate (). Shortly after, the approach was applied to the degradation of fatty acids in the laboratory of Adolf Lieben. However, it is named for Cläre Hunsdiecker and her husband Heinz Hunsdiecker, whose work in the 1930s developed it into a general method. Several reviews have been published, and a catalytic approach has been developed. : History Alexander Borodin first observed the reaction in 1861 when h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krapcho Decarboxylation
The Krapcho decarboxylation is the chemical reaction of esters with halide anions. The ester must contain an electron-withdrawing group in the beta position, such as β-ketoesters, malonic esters, α-cyanoesters, or α-sulfonylesters. It works best with methyl esters, since methyl groups are more susceptible to SN2-reaction than are, say, ethyl groups. The byproducts of this decarbomethoxylation are chloromethane Chloromethane, also called methyl chloride, Refrigerant-40, R-40 or HCC 40, is an organic compound with the chemical formula . One of the haloalkanes, it is a colorless, odorless, flammable gas. Methyl chloride is a crucial reagent in industrial ... and CO2. They are lost as gases, which helps drive the reaction. The Krapcho decarboxylation is a useful way to manipulate malonic esters because it cleaves only one of the two ester groups. The apparent alternative, base hydrolysis followed by decarboxylation, requires a subsequent step to regenerate the ester. : Re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tsuji–Trost Reaction
The Tsuji–Trost reaction (also called the Trost allylic alkylation or allylic alkylation) is a palladium- catalysed substitution reaction involving a substrate that contains a leaving group in an allylic position. The palladium catalyst first coordinates with the allyl group and then undergoes oxidative addition, forming the -allyl complex. This allyl complex can then be attacked by a nucleophile, resulting in the substituted product. This work was first pioneered by in 1965 and, later, adapted by Barry Trost in 1973 with the introduction of phosphine ligands. The scope of this reaction has been expanded to many different carbon, nitrogen, and oxygen-based nucleophiles, many different leaving groups, many different phosphorus, nitrogen, and sulfur-based ligands, and many different metals (although palladium is still preferred). The introduction of phosphine ligands led to improved reactivity and numerous asymmetric allylic alkylation strategies. Many of these strategies are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation Reaction
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases ( EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the corresponding aryl anion, which in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Complex
Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures. Examples The allyl ligand is commonly found in organometallic chemistry. Most commonly, allyl ligands bind to metals via all three carbon atoms, the η3-binding mode. The η3-allyl group is classified as an LX-type ligand in the Green LXZ ligand classification scheme, serving as a 3e– donor using neutral electron counting and 4e– donor using ionic electron counting. More common are complexes with allyl and other ligands. Examples include (η3-allyl)Mn(CO)4 and CpPd(allyl). Homoleptic complexes * bis(allyl)nickel * bis(allyl)palladium * bis(allyl)platinum *tris(allyl)chromium * tris(allyl)rhodium * tris(allyl)iridium Synthetic methods Allyl complexes are often generated by o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |