|
Dabrafenib
Dabrafenib, sold under the brand name Tafinlar & Rafinlar ( both by Novartis) among others, is a medication for the treatment of cancers associated with a mutated version of the gene BRAF. Dabrafenib acts as an inhibitor of the associated enzyme B-Raf, which plays a role in the regulation of cell growth. Dabrafenib has clinical activity with a manageable safety profile in clinical trials of phase 1 and 2 in patients with BRAF (V600)-mutated metastatic melanoma. Approvals and indications The US Food and Drug Administration initially approved dabrafenib as a single agent treatment for patients with BRAF V600E mutation-positive advanced melanoma on May 29, 2013. Dabrafenib was approved for use in the European Union in August 2013. Clinical trial data demonstrated that resistance to dabrafenib and other BRAF inhibitors occurs within six to seven months. To overcome this resistance, the BRAF inhibitor dabrafenib was combined with the MEK inhibitor trametinib. On January 8, 2014, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanoma
Melanoma, also redundantly known as malignant melanoma, is a type of skin cancer that develops from the pigment-producing cells known as melanocytes. Melanomas typically occur in the skin, but may rarely occur in the mouth, intestines, or eye ( uveal melanoma). In women, they most commonly occur on the legs, while in men, they most commonly occur on the back. About 25% of melanomas develop from moles. Changes in a mole that can indicate melanoma include an increase in size, irregular edges, change in color, itchiness, or skin breakdown. The primary cause of melanoma is ultraviolet light (UV) exposure in those with low levels of the skin pigment melanin. The UV light may be from the sun or other sources, such as tanning devices. Those with many moles, a history of affected family members, and poor immune function are at greater risk. A number of rare genetic conditions, such as xeroderma pigmentosum, also increase the risk. Diagnosis is by biopsy and analysis of any sk ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trametinib
Trametinib, sold under the brand name Mekinist among others, is an anticancer medication used for the treatment of melanoma. It is a MEK inhibitor drug with anti-cancer activity. It inhibits MEK1 and MEK2. Trametinib had good results for metastatic melanoma carrying the BRAF V600E mutation in a phase III clinical trial. In this mutation, the amino acid valine (V) at position 600 within the BRAF protein has become replaced by glutamic acid (E) making the mutant BRAF protein constitutively active. In May 2013, trametinib was approved as a single-agent by the US Food and Drug Administration for the treatment of people with V600E mutated metastatic melanoma. Clinical trial data demonstrated that resistance to single-agent trametinib often occurs within 6 to 7 months. To overcome this, trametinib was combined with the BRAF inhibitor dabrafenib. As a result of this research, on January 8, 2014, the FDA approved the combination of dabrafenib and trametinib for the treatment of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BRAF (gene)
BRAF is a human gene that encodes a protein called B-Raf. The gene is also referred to as proto-oncogene B-Raf and v-Raf murine sarcoma viral oncogene homolog B, while the protein is more formally known as serine/threonine-protein kinase B-Raf. The B-Raf protein is involved in sending signals inside cells which are involved in directing cell growth. In 2002, it was shown to be mutated in some human cancers. Certain other inherited ''BRAF'' mutations cause birth defects. Drugs that treat cancers driven by ''BRAF'' mutations have been developed. Two of these drugs, vemurafenib and dabrafenib are approved by FDA for treatment of late-stage melanoma. Vemurafenib was the first approved drug to come out of fragment-based drug discovery. Function B-Raf is a member of the Raf kinase family of growth signal transduction protein kinases. This protein plays a role in regulating the MAP kinase/ ERKs signaling pathway, which affects cell division, differentiation, and secretion. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
V600E
V600E is a mutation of the BRAF gene in which valine (V) is substituted by glutamic acid (E) at amino acid 600. It is a driver mutation in a proportion of certain diagnoses, including melanoma, hairy cell leukemia, papillary thyroid carcinoma, colorectal cancer, non-small-cell lung cancer, Langerhans cell histiocytosis, Erdheim–Chester disease (a non-Langerhans-cell histiocytosis) and ameloblastoma. The mechanism of the mutation is that the negative charge of the acidic glutamic acid residue causes it to be phosphomimetic. This mimics the phosphorylation of the nearby T599 threonine and S602 serine residues in the activation segment of BRAF, which are used to activate the wild type form of the protein. The glutamate residue of the mutant therefore functions to activate BRAF by inhibiting the interaction of the BRAF's glycine rich loop and activation segment, which would ordinarily be inhibitory. The loss of inhibition of BRAF leads to an increase in its basal activity ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4 Inducers
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs which are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will therefore either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprotein, i.e. a protein containing a heme group with an iron atom. In humans, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
National Center For Biotechnology Information
The National Center for Biotechnology Information (NCBI) is part of the United States National Library of Medicine (NLM), a branch of the National Institutes of Health (NIH). It is approved and funded by the government of the United States. The NCBI is located in Bethesda, Maryland, and was founded in 1988 through legislation sponsored by US Congressman Claude Pepper. The NCBI houses a series of databases relevant to biotechnology and biomedicine and is an important resource for bioinformatics tools and services. Major databases include GenBank for DNA sequences and PubMed, a bibliographic database for biomedical literature. Other databases include the NCBI Epigenomics database. All these databases are available online through the Entrez search engine. NCBI was directed by David Lipman, one of the original authors of the BLAST sequence alignment program and a widely respected figure in bioinformatics. GenBank NCBI had responsibility for making available the GenBan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tert-butyl Compounds
In organic chemistry, butyl is a four-carbon alkyl radical or substituent group with general chemical formula , derived from either of the two isomers (''n''-butane and isobutane) of butane. The isomer ''n''-butane can connect in two ways, giving rise to two "-butyl" groups: * If it connects at one of the two terminal carbon atoms, it is normal butyl or ''n''-butyl: (preferred IUPAC name: butyl) * If it connects at one of the non-terminal (internal) carbon atoms, it is secondary butyl or ''sec''-butyl: (preferred IUPAC name: butan-2-yl) The second isomer of butane, isobutane, can also connect in two ways, giving rise to two additional groups: * If it connects at one of the three terminal carbons, it is isobutyl: (preferred IUPAC name: 2-methylpropyl) * If it connects at the central carbon, it is tertiary butyl, ''tert''-butyl or ''t''-butyl: (preferred IUPAC name: ''tert''-butyl) Nomenclature According to IUPAC nomenclature, "isobutyl", "''sec''-butyl", and "''tert''- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from oil and water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch, Peer ''Modern fluoroorga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiazoles
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen. The term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1). Molecular and electronic structure Thiazoles are members of the azoles, heterocycles that include imidazoles and oxazoles. Thiazole can also be considered a functional group. Oxazoles are related compounds, with sulfur replaced by oxygen. Thiazoles are structurally similar to imidazoles, with the thiazole sulfur replaced by nitrogen. Thiazole rings are planar and aromatic. Thiazoles are characterized by larger pi-electron delocalization than the corresponding oxazoles and have therefore greater aromaticity. This aromaticity is evidenced by the chemical shift of the ring protons in proton NMR spectroscopy (between 7.27 and 8.77 ppm), clearl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
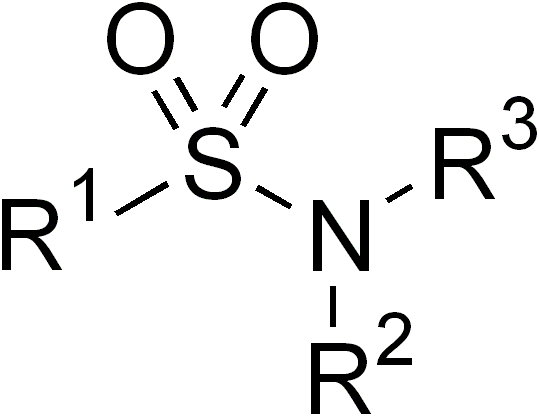
Sulfonamides
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |