|
Copper-free Click Chemistry
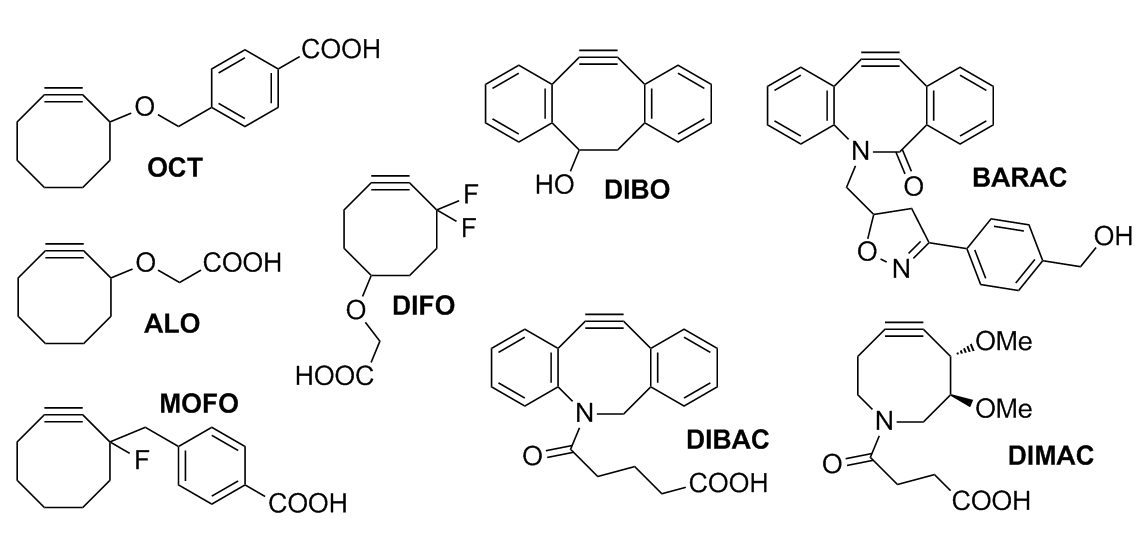
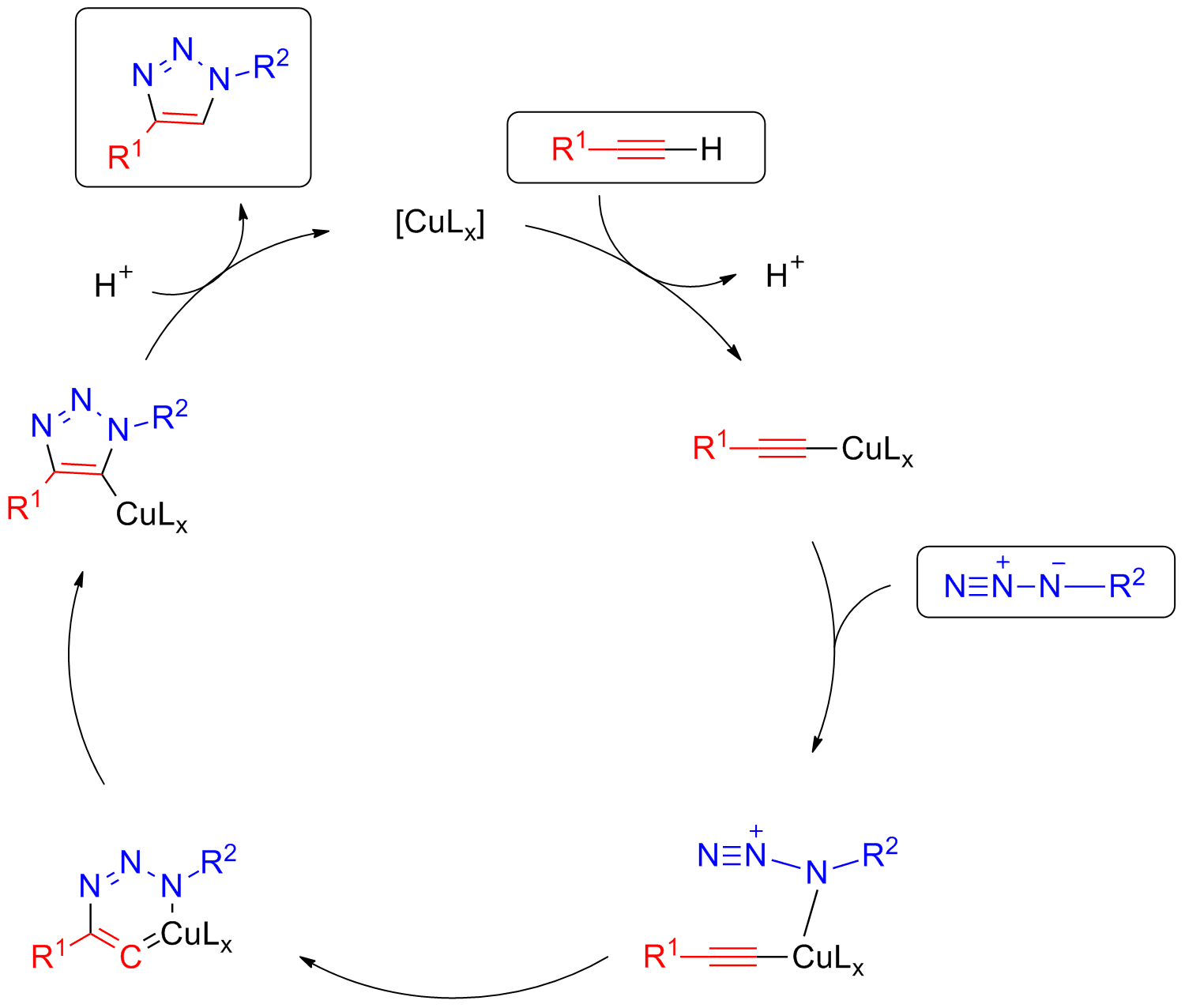
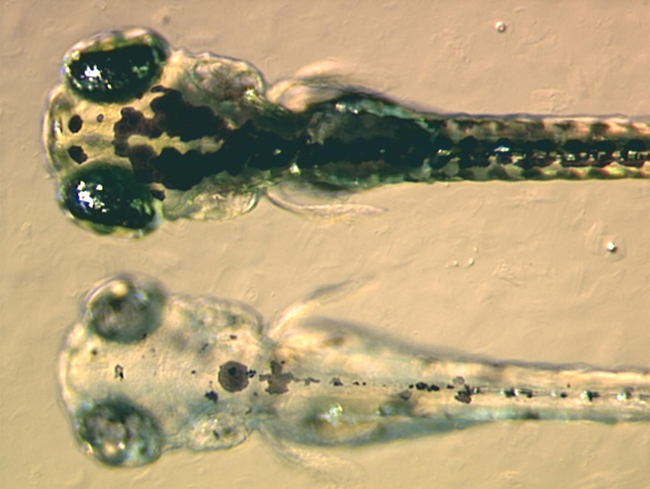
Copper-free click chemistry is a bio-orthogonal reaction as a variant of an azide-alkyne Huisgen cycloaddition. By eliminating cytotoxic copper catalysts, the reaction proceeds without live-cell toxicity. It was developed as a faster alternative to the Staudinger ligation with the first generation of Cu-free click chemistry, producing rate constants over 63 times faster. Although the reaction produces a regioisomeric mixture of triazoles, the lack of regioselectivity in the reaction is not a major concern for its applications in bioorthogonal chemistry. More regiospecific and less bio-orthogonal requirements are best served by the traditional Huisgen cycloaddition, especially given the low yield and synthetic difficulty of synthesizing a strained cyclooctyne (compared to the addition of a terminal alkyne). The bio-orthogonality of the reaction has allowed the Cu-free click reaction to be applied within cultured cells, live zebrafish, and mice. The absence of exogenous metal cat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioorthogonal Chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation. The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
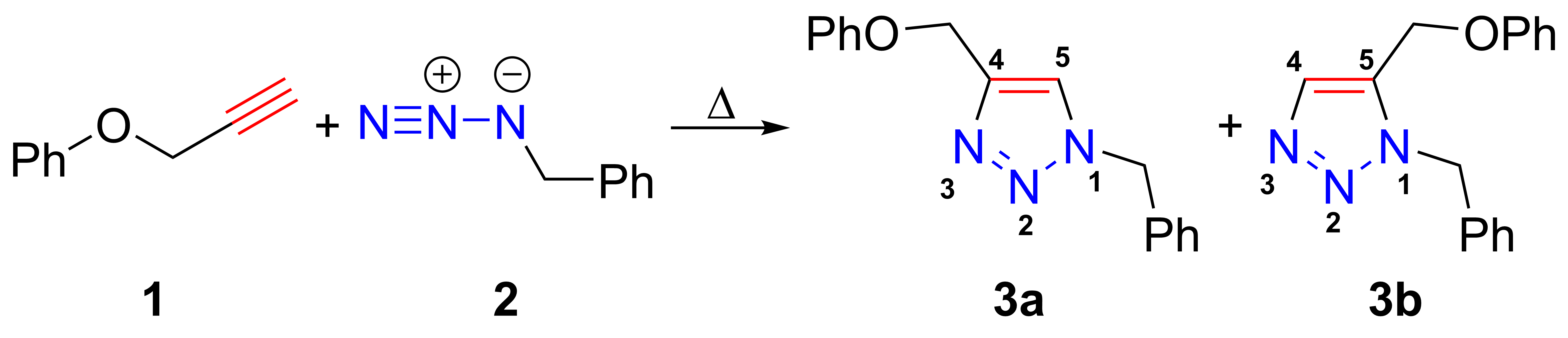
Azide-alkyne Huisgen Cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a change i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proceedings Of The National Academy Of Sciences
''Proceedings of the National Academy of Sciences of the United States of America'' (often abbreviated ''PNAS'' or ''PNAS USA'') is a peer-reviewed multidisciplinary scientific journal. It is the official journal of the National Academy of Sciences, published since 1915, and publishes original research, scientific reviews, commentaries, and letters. According to ''Journal Citation Reports'', the journal has a 2021 impact factor of 12.779. ''PNAS'' is the second most cited scientific journal, with more than 1.9 million cumulative citations from 2008 to 2018. In the mass media, ''PNAS'' has been described variously as "prestigious", "sedate", "renowned" and "high impact". ''PNAS'' is a delayed open access journal, with an embargo period of six months that can be bypassed for an author fee ( hybrid open access). Since September 2017, open access articles are published under a Creative Commons license. Since January 2019, ''PNAS'' has been online-only, although print issues are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Staudinger Ligation
The Staudinger reaction is a chemical reaction of an organic azide with a phosphine or phosphite produces an iminophosphorane. The reaction was discovered by and named after Hermann Staudinger. The reaction follows this stoichiometry: :R3P + R'N3 → R3P=NR' + N2 Staudinger reduction The Staudinger reduction is conducted in two steps. First phosphine imine-forming reaction is conducted involving treatment of the azide with the phosphine. The intermediate, e.g. triphenylphosphine phenylimide, is then subjected to hydrolysis to produce a phosphine oxide and an amine: :R3P=NR' + H2O → R3P=O + R'NH2 The overall conversion is a mild method of reducing an azide to an amine. Triphenylphosphine or tributylphosphine are most commonly used, yielding tributylphosphine oxide or triphenylphosphine oxide as a side product in addition to the desired amine. An example of a Staudinger reduction is the organic synthesis of the pinwheel compound 1,3,5-tris(aminomethyl)-2,4,6-triethylbenzene. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioorthogonal Chemistry
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes (also termed copper-free click chemistry), between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation. The use of bioorthogonal chemistry typically proceeds in two steps. First, a cellular substrate is modified with a bioorthogonal functional group (chemical r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Huisgen Cycloaddition
The azide-alkyne Huisgen cycloaddition is a 1,3-dipolar cycloaddition between an azide and a terminal or internal alkyne to give a 1,2,3-triazole. Rolf Huisgen was the first to understand the scope of this organic reaction. American chemist Karl Barry Sharpless has referred to this cycloaddition as "the cream of the crop" of click chemistry and "the premier example of a click reaction". In the reaction above azide 2 reacts neatly with alkyne 1 to afford the product triazole as a mixture of 1,4-adduct (3a) and 1,5-adduct (3b) at 98 °C in 18 hours. The standard 1,3-cycloaddition between an azide 1,3-dipole and an alkene as dipolarophile has largely been ignored due to lack of reactivity as a result of electron-poor olefins and elimination side reactions. Some success has been found with non-metal-catalyzed cycloadditions, such as the reactions using dipolarophiles that are electron-poor olefins or alkynes. Although azides are not the most reactive 1,3-dipole available for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclooctyne
Cyclooctyne is the cycloalkyne with a formula . Its molecule has a ring of 8 carbon atoms, connected by seven single bonds and one triple bond. Cyclooctene is the smallest cycloalkyne that is stable enough to be isolated, although the chemical is still highly reactive. The alkyne region of the structure attempts to adopt a linear molecular geometry, but the nature of the ring creates substantial ring strain. As a result, cyclooctyne and other compounds containing this ring structure readily react in ways that reduce the ring strain by converting the alkyne to a functional group that does not require linear geometry. An important application of this reactivity is in click chemistry, where cyclooctynes undergo cycloaddition reactions with azides or nitrones, forming triazoles or isoxazoline Isoxazolines are a class of five-membered heterocyclic chemical compounds, containing one atom each of oxygen and nitrogen which are located adjacent to one another. The ring was named in-line ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 121 picometers is much shorter than the C=C distance in alkenes (134 pm) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. The sigma bond contribute ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zebrafish
The zebrafish (''Danio rerio'') is a freshwater fish belonging to the minnow family ( Cyprinidae) of the order Cypriniformes. Native to South Asia, it is a popular aquarium fish, frequently sold under the trade name zebra danio (and thus often called a "tropical fish" although both tropical and subtropical). It is also found in private ponds. The zebrafish is an important and widely used vertebrate model organism in scientific research, for example in drug development, in particular pre-clinical development. It is also notable for its regenerative abilities, and has been modified by researchers to produce many transgenic strains. Taxonomy The zebrafish is a derived member of the genus '' Brachydanio'', of the family Cyprinidae. It has a sister-group relationship with ''Danio aesculapii''. Zebrafish are also closely related to the genus ''Devario'', as demonstrated by a phylogenetic tree of close species. Distribution Range The zebrafish is native to fresh water h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |