|
Concrete Sealer
Concrete sealers are applied to concrete to protect it from surface damage, corrosion, and staining. They either block the pores in the concrete to reduce absorption of water and salts or form an impermeable layer which prevents such materials from passing. Research from major concrete authorities, including the American Concrete Institute, Portland Cement Association, and the National Ready Mixed Concrete Association confirm that most concrete damage is attributable to surface moisture intrusion. The most pervasive form of concrete damage is surface scaling from freeze/thaw. Other forms of damage include alkali-silica reaction (ASR), chemical intrusion, and corrosion of steel reinforcements. Types In past decades attempts to protect concrete have included sealers ranging from wax to linseed oil. Today, high quality concrete sealers can block up to 99% of surface moisture. There are two main sealer categories: topical sealers (coatings) and penetrating sealers (reactive) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most widely used building material. Its usage worldwide, ton for ton, is twice that of steel, wood, plastics, and aluminum combined. Globally, the ready-mix concrete industry, the largest segment of the concrete market, is projected to exceed $600 billion in revenue by 2025. This widespread use results in a number of environmental impacts. Most notably, the production process for cement produces large volumes of greenhouse gas emissions, leading to net 8% of global emissions. Other environmental concerns include widespread illegal sand mining, impacts on the surrounding environment such as increased surface runoff or urban heat island effect, and potential public health implications from toxic ingredients. Significant research and development is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
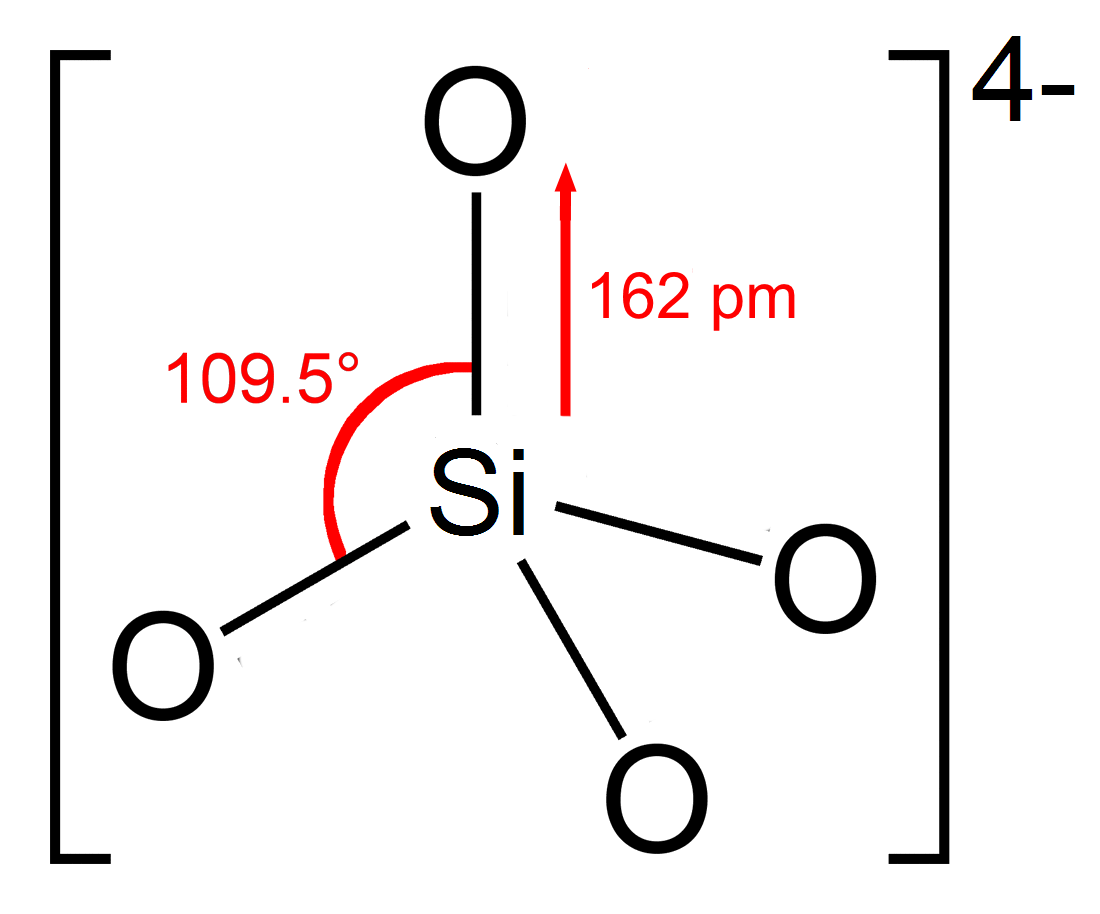
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedron whose corner ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosilicate
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials in 1904. In recognition of Kipping's achiev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Silicate
Lithium silicate may refer to: * Lithium metasilicate, an inorganic chemical compound with the formula Li2SiO3, used in calibrating thermocouples *Lithium orthosilicate Lithium orthosilicate is a compound with the chemical formula Li4SiO4. It is a white ceramic compound, which melts congruently at a temperature of . Lithium orthosilicate is of primary interest towards carbon dioxide capture, as this compound r ..., an inorganic chemical compound with the formula Li4SiO4, used as a carbon dioxide scrubber {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oleophobic
Lipophobicity, also sometimes called lipophobia (from the Greek λιποφοβία from λίπος ''lipos'' "fat" and φόβος ''phobos'' "fear"), is a chemical property of chemical compounds which means "fat rejection", literally "fear of fat". Lipophobic compounds are those not soluble in lipids or other non-polar solvents. From the other point of view, they do not absorb fats. "Oleophobic" (from the Latin ''oleum'' "oil", Greek ελαιοφοβικό ''eleophobico'' from έλαιο ''eleo'' "oil" and φόβος ''phobos'' "fear") refers to the physical property of a molecule that is seemingly repelled from oil. (Strictly speaking, there is no repulsive force involved; it is an absence of attraction.) The most common lipophobic substance is water. Fluorocarbons are also lipophobic/oleophobic in addition to being hydrophobic. Uses A lipophobic coating has been used on the touchscreens of Apple's iPhones since the 3GS, their iPads, Nokia's N9 and Lumia devices, the HTC HD2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobic
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. Hydrophobic is often used interchangeably with lipophilic, "fat-loving". However, the two terms are not synonymous. While hydrophobic substances are usually lipophilic, there are exceptions, suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Silicate Hydrate
Calcium silicate hydrate (or C-S-H) is the main product of the hydration of Portland cement and is primarily responsible for the strength in cement based materials (e.g. concrete). Preparation When water is added to cement, each of the compounds undergoes hydration and contributes to the final state of the concrete. Only calcium silicates contribute to the strength. Tricalcium silicate is responsible for most of the early strength (first 7 days). Dicalcium silicate, which reacts more slowly, only contributes to late strength. Calcium silicate hydrate (also shown as C-S-H) is a result of the reaction between the silicate phases of Portland cement and water. This reaction typically is expressed as: : Tricalcium silicate + water -> Calcium silicate hydrate + Calcium hydroxide + heat The stoichiometry of C-S-H in cement paste is variable and the state of chemically and physically bound water in its structure is not transparent, which is why "-" is used between C, S, and H. Synthet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Silicate
Sodium silicate is a generic name for chemical compounds with the formula or ·, such as sodium metasilicate , sodium orthosilicate , and sodium pyrosilicate . The anions are often polymeric. These compounds are generally colorless transparent solids or white powders, and soluble in water in various amounts. Sodium silicate is also the technical and common name for a mixture of such compounds, chiefly the metasilicate, also called waterglass, water glass, or liquid glass. The product has a wide variety of uses, including the formulation of cements, passive fire protection, textile and lumber processing, manufacture of refractory ceramics, as adhesives, and in the production of silica gel. The commercial product, available in water solution or in solid form, is often greenish or blue owing to the presence of iron-containing impurities. In industry, the various grades of sodium silicate are characterized by their SiO2:Na2O weight ratio (which can be converted to molar ratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Silicate
Lithium silicate may refer to: * Lithium metasilicate, an inorganic chemical compound with the formula Li2SiO3, used in calibrating thermocouples *Lithium orthosilicate Lithium orthosilicate is a compound with the chemical formula Li4SiO4. It is a white ceramic compound, which melts congruently at a temperature of . Lithium orthosilicate is of primary interest towards carbon dioxide capture, as this compound r ..., an inorganic chemical compound with the formula Li4SiO4, used as a carbon dioxide scrubber {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : Mg2Si + 4 HCl -> 2 MgCl2 + SiH4 It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : Si + 3 HCl -> HSiCl3 + H2 The trichlorosilane is then converted to a mixture of silane and silicon tetrachloride: : 4 HS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many structural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Novolak
Novolaks (sometimes: novolacs) are low molecular weight polymers derived from phenols and formaldehyde. They are related to Bakelite, which is more highly crosslinked. The term comes from Swedish "lack" for lacquer and Latin "novo" for new, since these materials were envisioned to replace natural lacquers such as copal resin. Typically novolaks are prepared by the condensation of phenol or a mixture of p- and m-cresol with formaldehyde (as formalin). The reaction is acid catalyzed. Oxalic acid is often used because it can be subsequently removed by thermal decomposition. Novolaks have a degree of polymerization of approximately 20-40. The branching density, determined by the processing conditions, m- vs p-cresol ratio, as well as CH2O/cresol ratio is typically around 15%. Novolaks are especially important in microelectronics where they are used as photoresist materials. They are also used as tackifier Tackifiers are chemical compounds used in formulating adhesives to incr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |