|
Comonomer
In polymer chemistry, a comonomer refers to a polymerizable precursor to a copolymer aside from the principal monomer. In some cases, only small amounts of a comonomer are employed, in other cases substantial amounts of comonomers are used. Furthermore, in some cases, the comonomers are statistically incorporated within the polymer chain, whereas in other cases, they aggregate. The distribution of comonomers is referred to as the " blockiness" of a copolymer. 1-Octene, 1-hexene, and 1-butene are used comonomers in the manufacture of polyethylenes. The advantages to such copolymers has led to a focus on catalysts that facilitate the incorporation of these comonomers, e.g., constrained geometry complexes. Comonomers are often employed to improve the plastification of polymeric materials, i.e. the flexibility of the polymer. Unlike traditional plasticizers, comonomers are not leachable. In other cases, comonomers are used to introduce crosslinking. Divinylbenzene Divinylbenz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
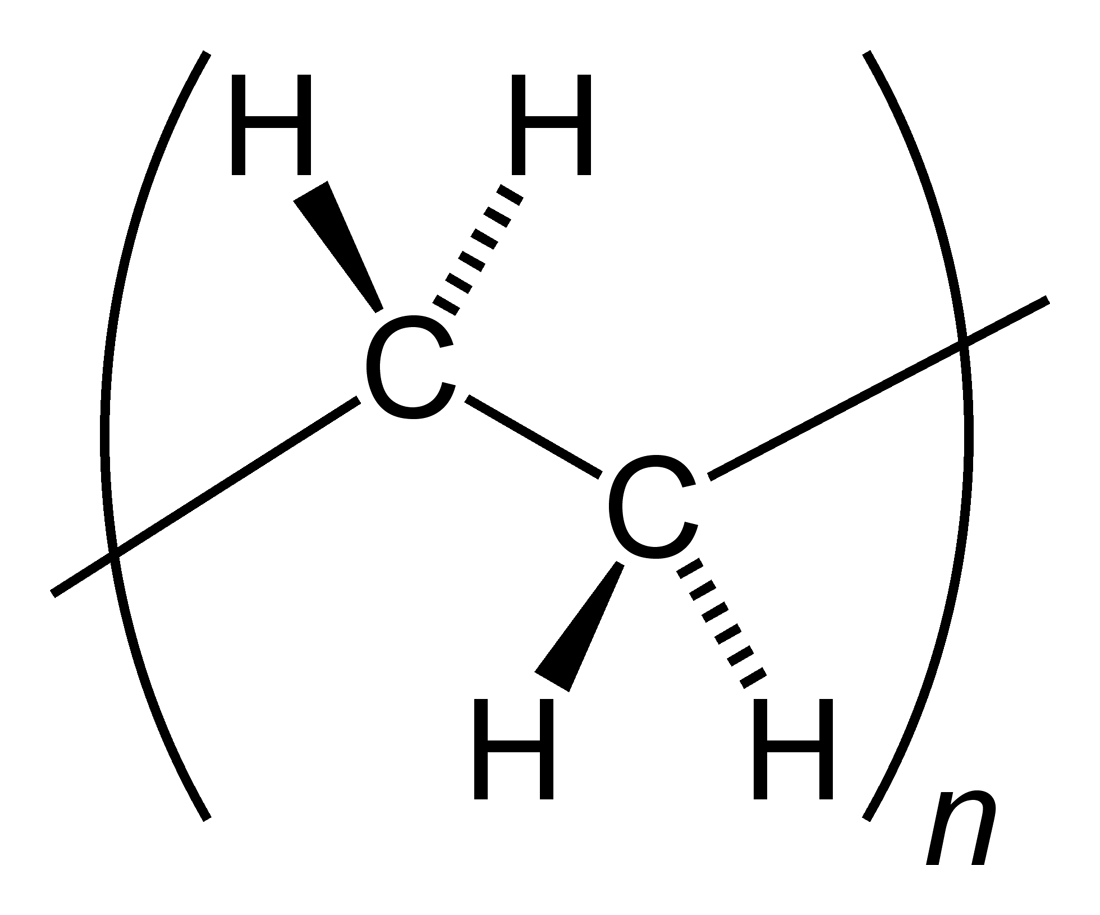
Polyethylene
Polyethylene or polythene (abbreviated PE; IUPAC name polyethene or poly(methylene)) is the most commonly produced plastic. It is a polymer, primarily used for packaging ( plastic bags, plastic films, geomembranes and containers including bottles, etc.). , over 100 million tonnes of polyethylene resins are being produced annually, accounting for 34% of the total plastics market. Many kinds of polyethylene are known, with most having the chemical formula (C2H4)''n''. PE is usually a mixture of similar polymers of ethylene, with various values of ''n''. It can be ''low-density'' or ''high-density'': low-density polyethylene is extruded using high pressure () and high temperature (), while high-density polyethylene is extruded using low pressure () and low temperature (). Polyethylene is usually thermoplastic, but it can be modified to become thermosetting instead, for example, in cross-linked polyethylene. History Polyethylene was first synthesized by the German chemist Hans ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monomers
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization. Classification Monomers can be classified in many ways. They can be subdivided into two broad classes, depending on the kind of the polymer that they form. Monomers that participate in condensation polymerization have a different stoichiometry than monomers that participate in addition polymerization: : Other classifications include: *natural vs synthetic monomers, e.g. glycine vs caprolactam, respectively *polar vs nonpolar monomers, e.g. vinyl acetate vs ethylene, respectively *cyclic vs linear, e.g. ethylene oxide vs ethylene glycol, respectively The polymerization of one kind of monomer gives a homopolymer. Many polymers are copolymers, meaning that they are derived from two different monomers. In the case of condensation polymerizations, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Octene
1-Octene is an organic compound with a formula CH2CHC6H13. The alkene is classified as a higher olefin and alpha-olefin, meaning that the double bond is located at the alpha (primary) position, endowing this compound with higher reactivity and thus useful chemical properties. 1-Octene is one of the important linear alpha olefins in industry. It is a colourless liquid. Synthesis In industry, 1-octene is commonly manufactured by two main routes: oligomerization of ethylene and by Fischer–Tropsch synthesis followed by purification. Another route to 1-octene that has been used commercially on a small scale is dehydration of alcohols. Prior to the 1970s, 1-octene was also manufactured by thermal cracking of waxes, whereas linear internal octenes were also manufactured by chlorination/dehydrochlorination of linear alkanes. There are five commercial processes that oligomerize ethylene to 1-octene. Four of these processes produce 1-octene as a part of a wide distribution of alpha-olefi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blockiness
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-butene
1-Butene (or 1-Butylene) is the organic compound with the formula CH3CH2CH=CH2. It is a colorless gas that is easily condensed to give a colorless liquid. It is classified as a linear alpha-olefin. It is one of the isomers of butene (butylene). It is a precursor to diverse products. Reactions Polymerization of 1-butene give polybutene, which is used to make piping for domestic plumbing. Its main application is as a comonomer in the production of certain kinds of polyethylene, such as linear low-density polyethylene (LLDPE). It has also been used as a precursor to polypropylene resins, butylene oxide, and butanone. Manufacturing 1-Butene is produced by separation from crude C4 refinery streams and by ethylene dimerization. The former affords a mixture of 1-and 2-butenes, while the latter affords only the terminal alkene. It is distilled to give a very high purity product. An estimated 12 billion kilograms were produced in 2011. See also *Butene *Dimer (chemistry) *Octene Oct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Constrained Geometry Complex
In organometallic chemistry, a "constrained geometry complex" (CGC) is a kind of catalyst used for the production of polyolefins such as polyethylene and polypropylene. The catalyst was one of the first major departures from metallocene-based catalysts and ushered in much innovation in the development of new plastics. Structure CGC complexes feature a pi-bonded moiety (e.g. cyclopentadienyl) linked to one of the other ligands on the same metal centre in such a way that the angle at this metal between the centroid of the pi-system and the additional ligand is smaller than in comparable unbridged complexes. More specifically, the term CGC was used for ansa-bridged cyclopentadienyl amido complexes, although the definition goes far beyond this class of compounds. The term CGC is frequently used in connection with other more or less related ligand systems that may or may not be isolobal and/or isoelectronic with the ansa-bridged cyclopentadienyl amido ligand system. Furthermore, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer Chemistry
Polymer chemistry is a sub-discipline of chemistry that focuses on the structures of chemicals, chemical synthesis, and chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry. Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules. However, polymer chemistry is typically related to synthetic and organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, such as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.Hans-Heinrich Moretto, Manfred Sch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Divinylbenzene
Divinylbenzene (DVB) consists of a benzene ring bonded to two vinyl groups. It is related to styrene (vinylbenzene) by the addition of a second vinyl group.CRC Handbook of Chemistry and Physics 65Th Ed. It is a colorless liquid manufactured by the thermal dehydrogenation of isomeric diethylbenzenes. Under synthesis conditions, ''o''-divinylbenzene converts to naphthalene and thus is not a component of the usual mixtures of DVB. Production and use It is produced by dehydrogenation of diethylbenzene: : C6H4(C2H5)2 → C6H4(C2H3)2 + 2 H2 Divinylbenzene is usually encountered as a 2:1 mixture of ''m''- and ''p''-divinylbenzene, containing also the corresponding isomers of ethylvinylbenzene. Styrene and divinylbenzene react to form the copolymer styrene-divinylbenzene, S-DVB or Sty-DVB. The resulting cross-linked polymer is mainly used for the production of ion exchange resin and Merrifield resin Merrifield Resin is a cross-linked polystyrene resin that carries a chloromet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concentrations have a less pleasant odor. Styrene is the precursor to polystyrene and several copolymers. Approximately 25 million tonnes of styrene were produced in 2010, increasing to around 35 million tonnes by 2018. Natural occurrence Styrene is named after storax balsam (often commercially sold as ''styrax''), the resin of Liquidambar trees of the Altingiaceae plant family. Styrene occurs naturally in small quantities in some plants and foods (cinnamon, coffee beans, balsam tree (other), balsam trees and peanuts) and is also found in coal tar. History In 1839, the German apothecary Eduard Simon isolated a volatile liquid from the resin (called ''storax'' or ''styrax'' (Latin)) of the Liquidambar styraciflua, American sweetgu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |