|
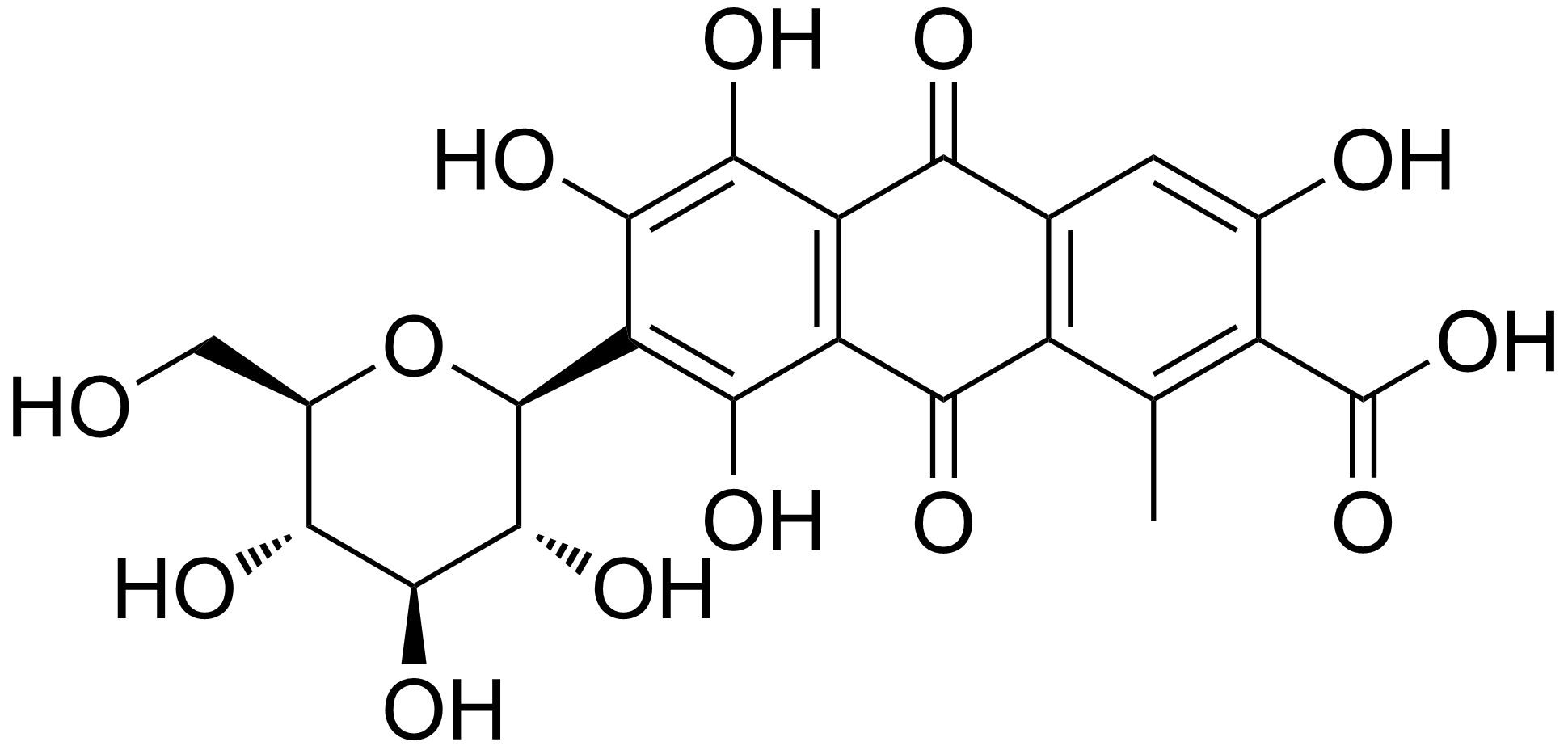
Carminic Acid
Carminic acid (C22H20O13) is a red glucosidal hydroxyanthrapurin that occurs naturally in some scale insects, such as the cochineal, Armenian cochineal, and Polish cochineal. The insects produce the acid as a deterrent to predators. An aluminum salt of carminic acid is the coloring agent in carmine, a pigment. Natives of Peru had been producing cochineal dyes for textiles since at least 700 CE. Synonyms are C.I. 75470 and C.I. Natural Red 4. The chemical structure of carminic acid consists of a core anthraquinone structure linked to a glucose sugar unit. Carminic acid was first synthesized in the laboratory by organic chemists in 1991. In 2018, researchers genetically engineered the microbe ''Aspergillus nidulans'' to produce carminic acid. It was previously thought that it contains α-D-glucopyranosyl residue, which was later redetermined to be the β-D-glucopyranosyl anomer. Harvesting from cochineals Carminic acid is commonly harvested from an American species scal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucoside
A glucoside is a glycoside that is derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is hydrolysed by purely chemical means, or decomposed by fermentation or enzymes. The name was originally given to plant products of this nature, in which the other part of the molecule was, in the greater number of cases, an aromatic aldehydic or phenolic compound (exceptions are Jinigrin and Jalapin or Scammonin). It has now been extended to include synthetic ethers, such as those obtained by acting on alcoholic glucose solutions with hydrochloric acid, and also the polysaccharoses, e.g. cane sugar, which appear to be ethers also. Although glucose is the most common sugar present in glucosides, many are known which yield rhamnose or iso-dulcite; these may be termed pentosides. Much attention has been given to the non-sugar parts (aglyca) of the molecules; the constitutions of many have been determined, and the compounds synthesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dactylopius Coccus (8410000864)
The cochineal ( , ; ''Dactylopius coccus'') is a scale insect in the suborder Sternorrhyncha, from which the natural dye carmine is derived. A primarily sessile parasite native to tropical and subtropical South America through North America (Mexico and the Southwest United States), this insect lives on cacti in the genus '' Opuntia'', feeding on plant moisture and nutrients. The insects are found on the pads of prickly pear cacti, collected by brushing them off the plants, and dried. The insect produces carminic acid that deters predation by other insects. Carminic acid, typically 17–24% of dried insects' weight, can be extracted from the body and eggs, then mixed with aluminium or calcium salts to make carmine dye, also known as cochineal. Today, carmine is primarily used as a colorant in food and in lipstick ( E120 or Natural Red 4). Carmine dye was used in the Americas for coloring fabrics and became an important export good in the 16th century during the coloni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
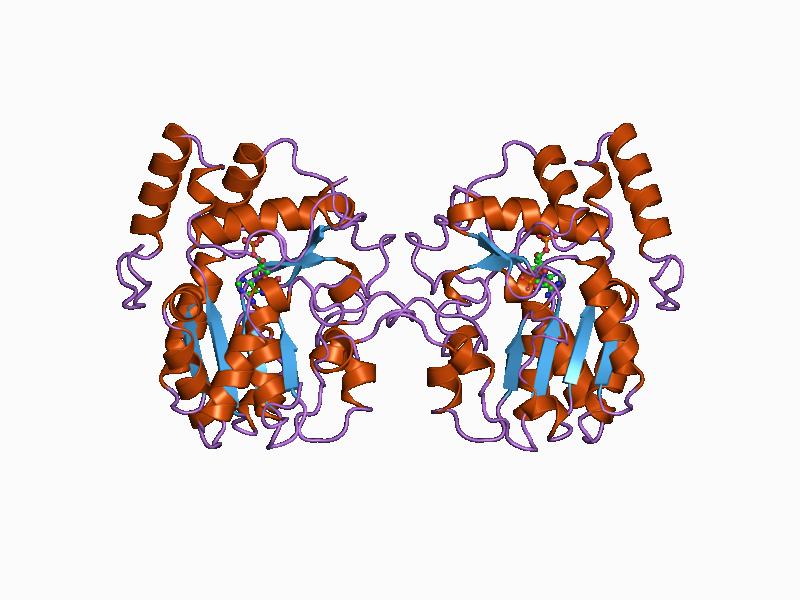
Acyl Carrier Protein
The acyl carrier protein (ACP) is a cofactor of both fatty acid and polyketide biosynthesis machinery. It is one of the most abundant proteins in cells of ''E. coli.'' In both cases, the growing chain is bound to the ACP via a thioester derived from the distal thiol of a 4'-phosphopantetheine moiety. Structure The ACPs are small negatively charged α-helical bundle proteins with a high degree of structural and amino acid similarity. The structures of a number of acyl carrier proteins have been solved using various NMR and crystallography techniques. The ACPs are related in structure and mechanism to the peptidyl carrier proteins (PCP) from nonribosomal peptide synthases. Biosynthesis Subsequent to the expression of the inactive ''apo'' ACP, the 4'-phosphopantetheine moiety is attached to a serine residue. This coupling is mediated by acyl carrier protein synthase (ACPS), a 4'-phosphopantetheinyl transferase. 4'-Phosphopantetheine is a prosthetic group of several acyl carrier pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transferase
A transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved in hundreds of different biochemical pathways throughout biology, and are integral to some of life's most important processes. Transferases are involved in myriad reactions in the cell. Three examples of these reactions are the activity of coenzyme A (CoA) transferase, which transfers thiol esters, the action of N-acetyltransferase, which is part of the pathway that metabolizes tryptophan, and the regulation of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl CoA. Transferases are also utilized during translation. In this case, an amino acid chain is the functional group transferred by a peptidyl transferase. The transfer involves the removal of the growing amino acid chain from the tRNA molecule in the A-site of the ribosome and its subse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shikimate Pathway
The shikimate pathway (shikimic acid pathway) is a seven-step metabolic pathway used by bacteria, archaea, fungi, algae, some protozoans, and plants for the biosynthesis of folates and aromatic amino acids (tryptophan, phenylalanine, and tyrosine). This pathway is not found in animal cells. The seven enzymes involved in the shikimate pathway are DAHP synthase, 3-dehydroquinate synthase, 3-dehydroquinate dehydratase, shikimate dehydrogenase, shikimate kinase, EPSP synthase, and chorismate synthase. The pathway starts with two substrates, phosphoenol pyruvate and erythrose-4-phosphate, and ends with chorismate, a substrate for the three aromatic amino acids. The fifth enzyme involved is the shikimate kinase, an enzyme that catalyzes the ATP-dependent phosphorylation of shikimate to form shikimate 3-phosphate (shown in the figure below). Shikimate 3-phosphate is then coupled with phosphoenol pyruvate to give 5-enolpyruvylshikimate-3-phosphate via the enzyme 5-enolpyruvylshikimate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Metabolite
Secondary metabolites, also called specialised metabolites, toxins, secondary products, or natural products, are organic compounds produced by any lifeform, e.g. bacteria, fungi, animals, or plants, which are not directly involved in the normal growth, development, or reproduction of the organism. Instead, they generally mediate ecological interactions, which may produce a selective advantage for the organism by increasing its survivability or fecundity. Specific secondary metabolites are often restricted to a narrow set of species within a phylogenetic group. Secondary metabolites often play an important role in plant defense against herbivory and other interspecies defenses. Humans use secondary metabolites as medicines, flavourings, pigments, and recreational drugs. The term secondary metabolite was first coined by Albrecht Kossel, a 1910 Nobel Prize laureate for medicine and physiology in 1910. 30 years later a Polish botanist Friedrich Czapek described secondary metabolit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyketide
Polyketides are a class of natural products derived from a precursor molecule consisting of a chain of alternating ketone (or reduced forms of a ketone) and methylene groups: (-CO-CH2-). First studied in the early 20th century, discovery, biosynthesis, and application of polyketides has evolved. It is a large and diverse group of secondary metabolites caused by its complex biosynthesis which resembles that of fatty acid synthesis. Because of this diversity, polyketides can have various medicinal, agricultural, and industrial applications. Many polyketides are medicinal or exhibit acute toxicity. Biotechnology has enabled discovery of more naturally-occurring polyketides and evolution of new polyketides with novel or improved bioactivity. History Naturally produced polyketides by various plants and organisms have been used by humans since before studies on them began in the 19th and 20th century. In 1893, J. Norman Collie synthesized detectable amounts of orcinol by heating dehy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis Of Carminic Acid
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Laetilia Coccidivora
''Laetilia coccidivora'', the scale-feeding snout moth, is a species of snout moth The Pyralidae, commonly called pyralid moths, snout moths or grass moths, are a family of Lepidoptera in the ditrysian superfamily Pyraloidea. In many (particularly older) classifications, the grass moths (Crambidae) are included in the Pyralida ... in the genus ''Laetilia''. It was described by John Henry Comstock in 1879. It is found in the southern United States, including California, Florida, Maryland, North Carolina, Ohio, Oklahoma, Texas and West Virginia. The wingspan is 10–17 mm. The larvae are predatory on Coccidae species. They feed on the eggs and young. It uses carminic acid, acquired from its prey, as a defence against its own predators. References Moths described in 1879 Phycitini {{Phycitini-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cochineal Drawing
The cochineal ( , ; ''Dactylopius coccus'') is a scale insect in the suborder Sternorrhyncha, from which the natural dye carmine is derived. A primarily sessile parasite native to tropical and subtropical South America through North America (Mexico and the Southwest United States), this insect lives on cacti in the genus '' Opuntia'', feeding on plant moisture and nutrients. The insects are found on the pads of prickly pear cacti, collected by brushing them off the plants, and dried. The insect produces carminic acid that deters predation by other insects. Carminic acid, typically 17–24% of dried insects' weight, can be extracted from the body and eggs, then mixed with aluminium or calcium salts to make carmine dye, also known as cochineal. Today, carmine is primarily used as a colorant in food and in lipstick ( E120 or Natural Red 4). Carmine dye was used in the Americas for coloring fabrics and became an important export good in the 16th century during the coloni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_04_ies.jpg)