|
Carboxyatractyloside
Carboxyatractyloside (CATR) is a highly toxic diterpene glycoside that inhibits the ADP/ATP translocase. It is about 10 times more potent than its analog atractyloside. While atractyloside is effective in the inhibition of oxidative phosphorylation, carboxyatractyloside is considered to be more effective. The effects of carboxyatractyloside on the ADP/ATP translocase are not reversed by increasing the concentration of adenine nucleotides, unlike its counterpart atractyloside. Carboxyatractyloside behavior resembles bongkrekic acid while in the mitochondria. Carboxyatractyloside is poisonous to humans as well as livestock, including cows and horses. Symptoms of carboxyatractyloside poisoning may include abdominal pain, nausea and vomiting, drowsiness, palpitations, sweating and trouble breathing. In severe cases, convulsions, liver failure and loss of consciousness may develop, which can lead to death. Carboxyatractyloside can be found in ''Xanthium'' species plants, including ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADP/ATP Translocase
Adenine nucleotide translocator (ANT), also known as the ADP/ATP translocase (ANT), ADP/ATP carrier protein (AAC) or mitochondrial ADP/ATP carrier, exchanges free ATP with free ADP across the inner mitochondrial membrane. ANT is the most abundant protein in the inner mitochondrial membrane and belongs to mitochondrial carrier family. Free ADP is transported from the cytoplasm to the mitochondrial matrix, while ATP produced from oxidative phosphorylation is transported from the mitochondrial matrix to the cytoplasm, thus providing the cells with its main energy currency. ADP/ATP translocases are exclusive to eukaryotes and are thought to have evolved during eukaryogenesis. Human cells express four ADP/ATP translocases: SLC25A4, SLC25A5, SLC25A6 and SLC25A31, which constitute more than 10% of the protein in the inner mitochondrial membrane. These proteins are classified under the mitochondrial carrier superfamily. Types In humans, there exist three paraologous ANT isoforms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atractyloside
Atractyloside (ATR) is a natural, toxic glycoside present in numerous plant species worldwide in the daisy family including '' Atractylis gummifera'' and '' Callilepis laureola'', and it's used for a variety of therapeutic, religious, and toxic purposes. Exposure to ATR via ingestion or physical contact is toxic and can be fatal for both humans and animals, especially by kidney and liver failure. ATR acts as an effective ADP/ATP translocase inhibitor which eventually halts ADP and ATP exchange and the cell dies due to lack of energy. Historically, atractyloside poisoning has been challenging to verify and quantify toxicologically, though recent literature has described such methods within acceptable standards of forensic science. Sources Atractyloside is found in numerous plant species in the daisy family e.g. '' Atractylis gummifera'', '' Callilepis laureola'', '' Xanthium strumarium'', '' Iphiona alsoeri'', '' Pascalia glauca'', '' Wedelia glauca'', and '' Iphiona aucheri'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atractylis Gummifera
''Chamaeleon gummifer'', also known as distaff thistle or stemless atractylis, is a thistle in the ''Chamaeleon'' genus. Formerly, it was placed in the ''Atractylis'' genus. It is native to the Mediterranean basin, where it can be found in various habitats, including cultivated- or uncultivated fields and forests. It is a perennial herb producing a stemless, pinkish flower. The plant has a history of use in folk medicine, but it is very toxic due to the presence of atractyloside and carboxyatractyloside. Description ''Chamaeleon gummifer'' is a perennial thistle with a long rhizome extending up to 40 cm and spiky leaves emanating from its center. A pinkish inflorescence grows in the center, seen as a capitulum consisting of many small threadlike flowers. The inflorescence is surrounded by spiny bracts. Unusual compared to other thistles is the fact that the inflorescence of ''Chamaeleon gummifer'' does not grow on a stem. The ripe fruit of the plant may ooze a white or yellowi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthium
''Xanthium'' (cocklebur) is a genus of flowering plants in the tribe Heliantheae within the family Asteraceae, native to the Americas and eastern Asia and some parts of south Asia . Description Cockleburs are coarse, herbaceous annual plants growing to tall. The leaves are spirally arranged, with deeply toothed margins. Some species, notably ''Xanthium spinosum'', are also very thorny with long, slender spines at the leaf bases. The flower heads are of two types; One, in short terminal branches, produces only pollen. The other, in clusters in the axils of the leaves, produces seed. Unlike many other members of the family Asteraceae, whose seeds are airborne with a plume of silky hairs resembling miniature parachutes, cocklebur seeds are produced in a hard, spiny, globose or oval double-chambered, single-seeded bur long. It is covered with stiff, hooked spines, which stick to fur and clothing and can be quite difficult to detach. These burs are carried long distances fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthium Strumarium
''Xanthium strumarium'' (rough cocklebur, clotbur, common cocklebur, large cocklebur, woolgarie bur) is a species of annual plants of the family Asteraceae. Some sources claim it originates in southern Europe and Asia, but has been extensively naturalized elsewhere. Others, such as the Flora of China and Flora of North America, state it originates in the Americas but was an early introduction to Eurasia. Reproductive biology The species is monoecious, with the flowers borne in separate unisexual heads: staminate (male) heads situated above the pistillate (female) heads in the inflorescence. The pistillate heads consist of two pistillate flowers surrounded by a spiny involucre. Upon fruiting, these two flowers ripen into two brown to black achenes and they are completely enveloped by the involucre, which becomes a bur. The bur, being buoyant, easily disperses in the water for plants growing along waterways. However, the bur, with its hooked projections, is obviously adapted to disp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene Derivatives
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpene Glycosides
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
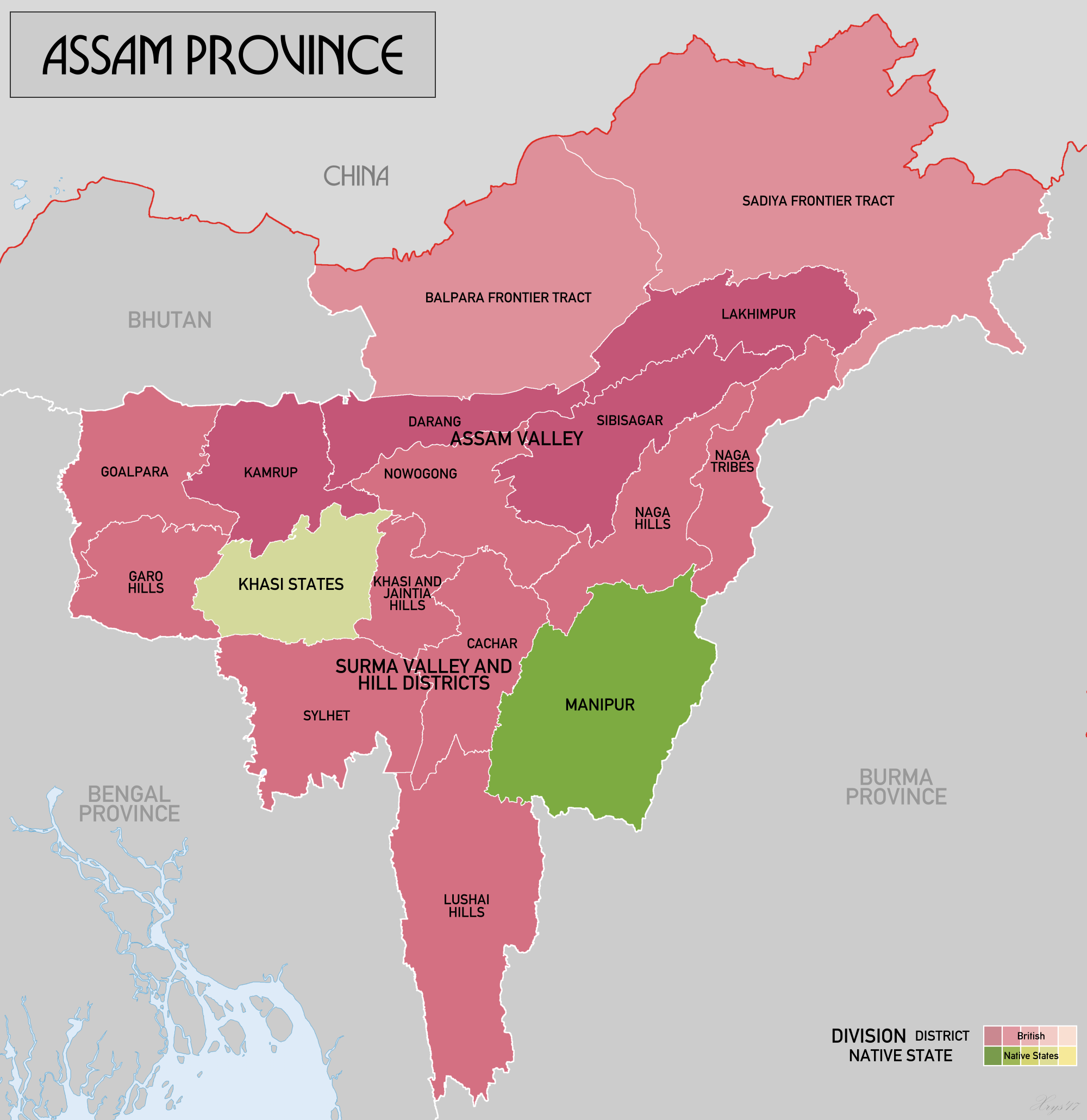
Sylhet Division
Sylhet Division ( bn, সিলেট বিভাগ) is the northeastern division of Bangladesh. It is bordered by the Indian states of Meghalaya, Assam and Tripura to the north, east and south respectively, and by the Bangladeshi divisions of Chittagong to the southwest and Dhaka and Mymensingh to the west. Prior to 1947, it included the subdivision of Karimganj (presently in Barak Valley, India). However, Karimganj (including the thanas of Badarpur, Patharkandi and Ratabari) was inexplicably severed from Sylhet by the Radcliffe Boundary Commission. According to Niharranjan Ray, it was partly due to a plea from a delegation led by Abdul Matlib Mazumdar. Etymology and names The name ''Sylhet'' is an anglicisation of ''Shilhot'' (শিলহট). Its origins seem to come from the Sanskrit words শিলা ''śilā'' (meaning 'stone') and হট্ট ''haṭṭa'' (meaning 'marketplace'). These words match the landscape and topography of the hilly region. The shila stones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpene
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycoside
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_drawn_over.png)