|
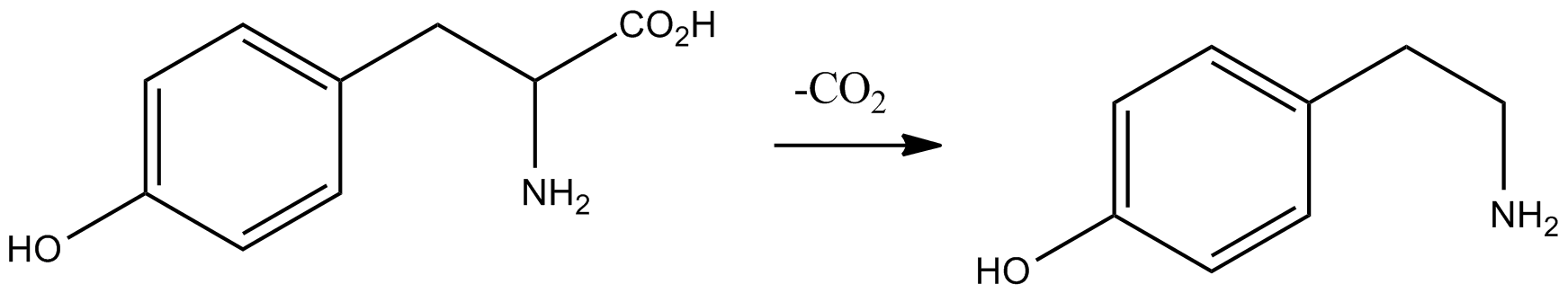
Candicine
Candicine is a naturally occurring organic compound that is a quaternary ammonium salt with a phenethylamine skeleton. It is the N,N,N-trimethyl derivative of the well-known biogenic amine tyramine, and, being a natural product with a positively charged nitrogen atom in its molecular structure, it is classed as an alkaloid. Although it is found in a variety of plants, including barley, its properties have not been extensively studied with modern techniques. Candicine is toxic after parenteral administration, producing symptoms of neuromuscular blocking drug, neuromuscular blockade; further details are given in the "Pharmacology" section below. Occurrence Candicine occurs in a variety of plants, notably the cacti. This alkaloid was first isolated from the Argentinian cactus ''Trichocereus candicans'' (now reclassified as ''Echinopsis candicans''), from which it derives its name, and from other ''Trichocereus'' species. ''T. candicans'' may contain up to 5% candicine, and is also a r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * strong or ag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hordenine
Hordenine is an alkaloid of the phenethylamine class that occurs naturally in a variety of plants, taking its name from one of the most common, barley (''Hordeum'' species). Chemically, hordenine is the ''N''-methyl derivative (chemistry), derivative of N-Methyltyramine, ''N''-methyltyramine, and the ''N'',''N''-dimethyl derivative of the well-known biogenic amine tyramine, from which it is biosynthetically derived and with which it shares some pharmacological properties (see below). , hordenine is widely sold as an ingredient of nutritional supplements, with the claims that it is a stimulant of the central nervous system, and has the ability to promote weight loss by enhancing metabolism. In experimental animals, given sufficiently large doses parenterally (by injection), hordenine does produce an increase in blood pressure, as well as other disturbances of the cardiovascular, respiratory, and nervous systems. These effects are generally not reproduced by oral administration of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, hydrogen cyanide), are not classified as organic compounds and are considered inorganic. Other than those just named, little consensus exists among chemists on precisely which carbon-containing compounds are excluded, making any rigorous definition of an organic compound elusive. Although organic compounds make up only a small percentage of Earth's crust, they are of central importance because all known life is based on organic compounds. Living t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amines
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
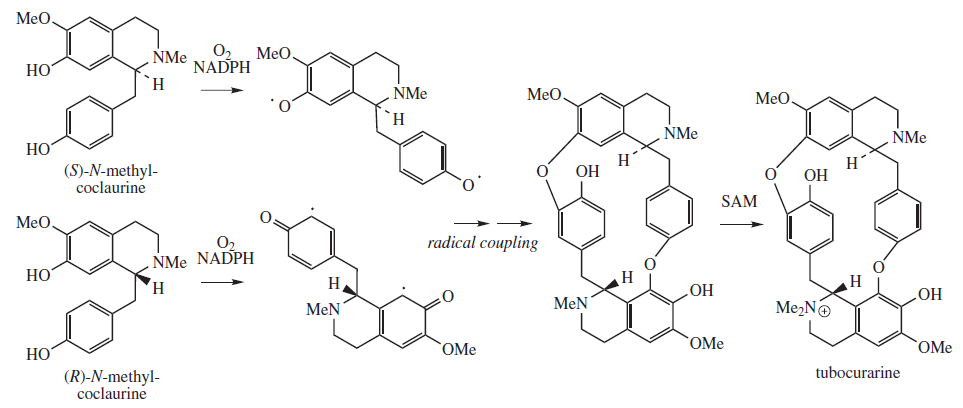
Tubocurarine
Tubocurarine (also known as ''d''-tubocurarine or DTC) is a toxic alkaloid historically known for its use as an arrow poison. In the mid-1900s, it was used in conjunction with an anesthetic to provide skeletal muscle relaxation during surgery or mechanical ventilation. It is now rarely used as an adjunct for clinical anesthesia because safer alternatives, such as cisatracurium and rocuronium, are available. History Tubocurarine is a naturally occurring mono-quaternary alkaloid obtained from the bark of the Menispermaceae, Menispermaceous South American plant ''Chondrodendron tomentosum'', a climbing vine known to the European world since the Spanish conquest of South America. Curare had been used as a source of arrow poison by South American natives to hunt animals, and they were able to eat the animals' contaminated flesh subsequently without any adverse effects because tubocurarine cannot easily cross mucous membranes. Thus, tubocurarine is effective only if given parenterally, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sartorius Muscle
The sartorius muscle () is the longest muscle in the human body. It is a long, thin, superficial muscle that runs down the length of the thigh in the Anterior compartment of thigh, anterior compartment. Structure The sartorius muscle originates from the anterior superior iliac spine, and part of the notch between the anterior superior iliac spine and anterior inferior iliac spine. It runs obliquely across the upper and anterior part of the thigh in an inferomedial direction. It passes behind the medial condyle of the femur to end in a tendon. This tendon curves anteriorly to join the tendons of the Gracilis muscle, gracilis and semitendinosus muscles in the pes anserinus (leg), pes anserinus, where it inserts into the superomedial surface of the tibia. Its upper portion forms the lateral border of the femoral triangle, and the point where it crosses Adductor longus muscle, adductor longus marks the apex of the triangle. Deep to sartorius and its fascia is the adductor canal, throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rectus Femoris Muscle
The rectus femoris muscle is one of the four quadriceps muscles of the human body. The others are the vastus medialis, the vastus intermedius (deep to the rectus femoris), and the vastus lateralis. All four parts of the quadriceps muscle attach to the patella (knee cap) by the quadriceps tendon. The rectus femoris is situated in the middle of the front of the thigh; it is fusiform in shape, and its superficial fibers are arranged in a bipenniform manner, the deep fibers running straight ( la, rectus) down to the deep aponeurosis. Its functions are to flex the thigh at the hip joint and to extend the leg at the knee joint. Structure It arises by two tendons: one, the anterior or straight, from the anterior inferior iliac spine; the other, the posterior or reflected, from a groove above the rim of the acetabulum. The two unite at an acute angle and spread into an aponeurosis that is prolonged downward on the anterior surface of the muscle, and from this the muscular fibers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Curare
Curare ( /kʊˈrɑːri/ or /kjʊˈrɑːri/; ''koo-rah-ree'' or ''kyoo-rah-ree'') is a common name for various alkaloid arrow poisons originating from plant extracts. Used as a paralyzing agent by indigenous peoples in Central and South America for hunting and for therapeutic purposes, curare only becomes active when it contaminates a wound. These poisons cause weakness of the skeletal muscles and, when administered in a sufficient dose, eventual death by asphyxiation due to paralysis of the diaphragm. Curare is prepared by boiling the bark of one of the dozens of plant sources, leaving a dark, heavy paste that can be applied to arrow or dart heads. In medicine, curare has been used as a treatment for tetanus or strychnine poisoning and as a paralyzing agent for surgical procedures. History The word 'curare' is derived from ''wurari'', from the Carib language of the Macusi of Guyana. It has its origins in the Carib phrase "mawa cure" meaning of the Mawa vine, scienti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Muscarinic
Muscarinic acetylcholine receptors, or mAChRs, are acetylcholine receptors that form G protein-coupled receptor complexes in the cell membranes of certain neurons and other cells. They play several roles, including acting as the main end-receptor stimulated by acetylcholine released from postganglionic fibers in the parasympathetic nervous system. Muscarinic receptors are so named because they are more sensitive to muscarine than to nicotine. Their counterparts are nicotinic acetylcholine receptors (nAChRs), receptor ion channels that are also important in the autonomic nervous system. Many drugs and other substances (for example pilocarpine and scopolamine) manipulate these two distinct receptors by acting as selective agonists or antagonists. Function Acetylcholine (ACh) is a neurotransmitter found in the brain, neuromuscular junctions and the autonomic ganglia. Muscarinic receptors are used in the following roles: Recovery receptors ACh is always used as the neuro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sparteine
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in ''Lupinus mutabilis'', and is thought to chelate the bivalent cations calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs. It is also used as a chiral ligand in organic chemistry, especially in syntheses involving organolithium reagents. Biosynthesis Sparteine is a lupin alkaloid containing a tetracyclic bis-quinolizidine ring system derived from three C5 chains of lysine, or more specifically, L-lysine. The first intermediate in the biosynthesis is cadaverine, the decarboxylation product of lysine catalyzed by the enzyme lysine decarboxylase (LDC). Three units of cadaverine are used to form the quinolizidine skeleton. The mechanism of formation has been studied enzymatically, as well as with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atropine
Atropine is a tropane alkaloid and anticholinergic medication used to treat certain types of nerve agent and pesticide poisonings as well as some types of slow heart rate, and to decrease saliva production during surgery. It is typically given intravenously or by injection into a muscle. Eye drops are also available which are used to treat uveitis and early amblyopia. The intravenous solution usually begins working within a minute and lasts half an hour to an hour. Large doses may be required to treat some poisonings. Common side effects include a dry mouth, large pupils, urinary retention, constipation, and a fast heart rate. It should generally not be used in people with angle closure glaucoma. While there is no evidence that its use during pregnancy causes birth defects, that has not been well studied. It is likely safe during breastfeeding. It is an antimuscarinic (a type of anticholinergic) that works by inhibiting the parasympathetic nervous system. Atropine occurs n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |