|
Copper Thiocyanate
Copper(I) thiocyanate (or cuprous thiocyanate) is a coordination polymer with formula CuSCN. It is an air-stable, white solid used as a precursor for the preparation of other thiocyanate salts. Structure At least two Polymorphism (materials science), polymorphs have been characterized by X-ray crystallography. They both feature copper(I) in a characteristic tetrahedral coordination geometry. The sulfur end of the SCN- ligand is triply bridging ligand, bridging so that the coordination sphere for copper is CuS3N.Smith, D. L.; Saunders, V. I. "Preparation and Structure Refinement of the 2H Polytype of beta-Copper(I) Thiocyanate" Acta Crystallographica B, 1982, volume 38, 907-909. Synthesis Copper(I) thiocyanate forms from the spontaneous decomposition of black copper(II) thiocyanate, releasing thiocyanogen, especially when heated. It is also formed from copper(II) thiocyanate under water, releasing (among others) thiocyanic acid and the highly poisonous hydrogen cyanide. It is c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Iodide
Copper(I) iodide is the inorganic compound with the formula CuI. It is also known as cuprous iodide. It is useful in a variety of applications ranging from organic synthesis to cloud seeding. Copper(I) iodide is white, but samples often appear tan or even, when found in nature as rare mineral marshite, reddish brown, but such color is due to the presence of impurities. It is common for samples of iodide-containing compounds to become discolored due to the facile aerobic oxidation of the iodide anion to molecular iodine. Structure Copper(I) iodide, like most binary (containing only two elements) metal halides, is an inorganic polymer. It has a rich phase diagram, meaning that it exists in several crystalline forms. It adopts a zinc blende structure below 390 °C (γ-CuI), a wurtzite structure between 390 and 440 °C (β-CuI), and a rock salt structure above 440 °C (α-CuI). The ions are tetrahedrally coordinated when in the zinc blende or the wurtzite struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconductor
A semiconductor is a material which has an electrical resistivity and conductivity, electrical conductivity value falling between that of a electrical conductor, conductor, such as copper, and an insulator (electricity), insulator, such as glass. Its electrical resistivity and conductivity, resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities ("doping (semiconductor), doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cuprous Oxide
Copper(I) oxide or cuprous oxide is the inorganic compound with the formula Cu2O. It is one of the principal oxides of copper, the other being or copper(II) oxide or cupric oxide (CuO). This red-coloured solid is a component of some antifouling paints. The compound can appear either yellow or red, depending on the size of the particles. Copper(I) oxide is found as the reddish mineral cuprite. Preparation Copper(I) oxide may be produced by several methods. Most straightforwardly, it arises via the oxidation of copper metal: : 4 Cu + O2 → 2 Cu2O Additives such as water and acids affect the rate of this process as well as the further oxidation to copper(II) oxides. It is also produced commercially by reduction of copper(II) solutions with sulfur dioxide. Reactions Aqueous cuprous chloride solutions react with base to give the same material. In all cases, the color is highly sensitive to the procedural details. Formation of copper(I) oxide is the basis of the Fehlin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anti-fouling Paint
Anti-fouling paint is a specialized category of coatings applied as the outer (outboard) layer to the hull of a ship or boat, to slow the growth of and facilitate detachment of subaquatic organisms that attach to the hull and can affect a vessel's performance and durability. It falls into a category of commercially available underwater hull paints, also known as ''bottom paints''. Anti-fouling paints are often applied as one component of multi-layer coating systems which may have other functions in addition to their antifouling properties, such as acting as a barrier against corrosion on metal hulls that will degrade and weaken the metal, or improving the flow of water past the hull of a fishing vessel or high-performance racing yachts. Although commonly discussed as being applied to ships, antifouling paints are also of benefit in many other sectors such as off-shore structures and fish farms. History In the Age of Sail, sailing vessels suffered severely from the growth of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel(II) Oxide
Nickel is a chemical element with symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive but large pieces are slow to react with air under standard conditions because a passivation layer of nickel oxide forms on the surface that prevents further corrosion. Even so, pure native nickel is found in Earth's crust only in tiny amounts, usually in ultramafic rocks, and in the interiors of larger nickel–iron meteorites that were not exposed to oxygen when outside Earth's atmosphere. Meteoric nickel is found in combination with iron, a reflection of the origin of those elements as major end products of supernova nucleosynthesis. An iron–nickel mixture is thought to compose Earth's outer and inner cores. Use of nickel (as natural meteoric nickel–iron alloy) has been traced as far back as 3500 BCE. Nickel was first isolated and classified as an ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Siemens (unit)
The siemens (symbol: S) is the unit of electric conductance, electric susceptance, and electric admittance in the International System of Units (SI). Conductance, susceptance, and admittance are the reciprocals of resistance, reactance, and impedance respectively; hence one siemens is redundantly equal to the reciprocal of one ohm () and is also referred to as the '' mho''. The 14th General Conference on Weights and Measures approved the addition of the siemens as a derived unit in 1971. The unit is named after Ernst Werner von Siemens. In English, the same word ''siemens'' is used both for the singular and plural. Like other SI units named after people, the symbol is capitalized but the name of the unit is not. For the siemens this is particularly important to distinguish it from the second, symbol (lower case) s. The related property, electrical conductivity, is measured in units of siemens per metre (S/m). Definition For an element conducting direct current, electrica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dye-sensitized Solar Cell
A dye-sensitized solar cell (DSSC, DSC, DYSC or Grätzel cell) is a low-cost solar cell belonging to the group of thin film solar cells. It is based on a semiconductor formed between a photo-sensitized anode and an electrolyte, a '' photoelectrochemical'' system. The modern version of a dye solar cell, also known as the Grätzel cell, was originally co-invented in 1988 by Brian O'Regan and Michael Grätzel at UC Berkeley and this work was later developed by the aforementioned scientists at the École Polytechnique Fédérale de Lausanne (EPFL) until the publication of the first high efficiency DSSC in 1991. Michael Grätzel has been awarded the 2010 Millennium Technology Prize for this invention. The DSSC has a number of attractive features; it is simple to make using conventional roll-printing techniques, is semi-flexible and semi-transparent which offers a variety of uses not applicable to glass-based systems, and most of the materials used are low-cost. In practice it has p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
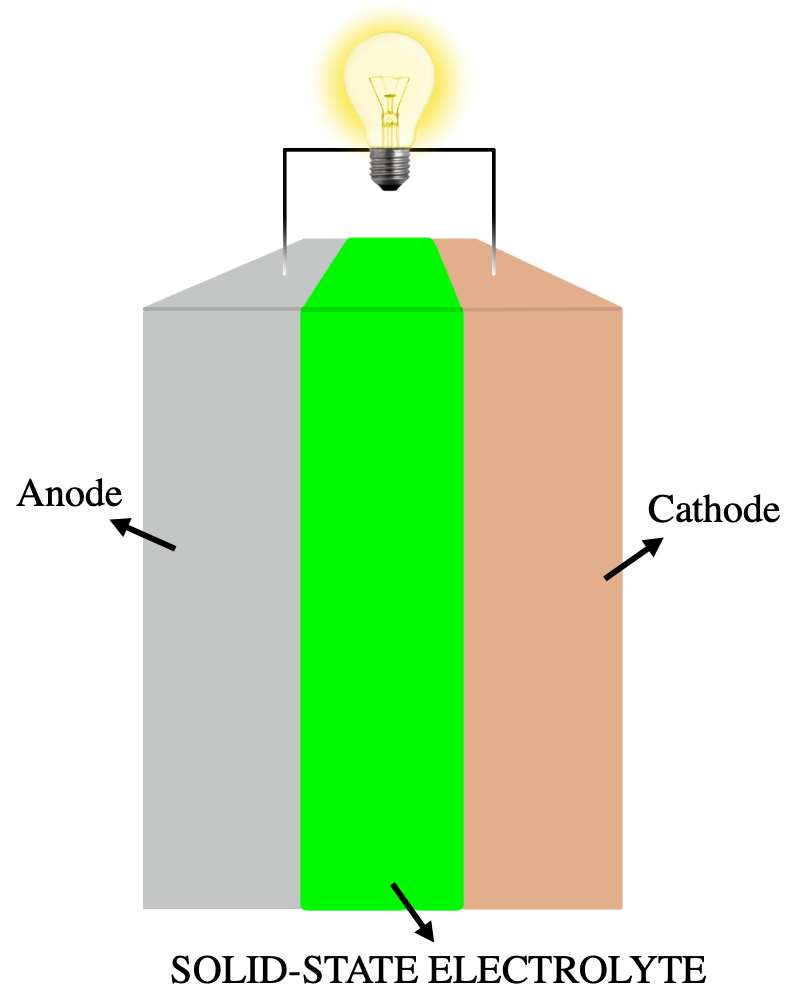
Solid-state Electrolyte
A solid-state electrolyte (SSE) is a solid Ionic conductivity (solid state), ionic conductor and electron-insulating electrolyte, material and it is the characteristic component of the solid-state battery. It is useful for applications in electrical energy storage (EES) in substitution of the liquid electrolytes found in particular in lithium-ion battery. The main advantages are the absolute safety, no issues of leakages of toxic organic liquid, organic solvents, low flammability, non-volatility, mechanical and thermal stability, easy processability, low self-discharge, higher achievable power density and cyclability. This makes possible, for example, the use of a lithium metal anode in a practical device, without the intrinsic limitations of a liquid electrolyte thanks to the property of lithium dendrite suppression in the presence of a solid-state electrolyte membrane. The utilization of a high capacity anode and low reduction potential, like lithium with a specific capacity of 38 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P-type Semiconductor
An extrinsic semiconductor is one that has been '' doped''; during manufacture of the semiconductor crystal a trace element or chemical called a doping agent has been incorporated chemically into the crystal, for the purpose of giving it different electrical properties than the pure semiconductor crystal, which is called an ''intrinsic semiconductor''. In an extrinsic semiconductor it is these foreign dopant atoms in the crystal lattice that mainly provide the charge carriers which carry electric current through the crystal. The doping agents used are of two types, resulting in two types of extrinsic semiconductor. An ''electron donor'' dopant is an atom which, when incorporated in the crystal, releases a mobile conduction electron into the crystal lattice. An extrinsic semiconductor which has been doped with electron donor atoms is called an n-type semiconductor, because the majority of charge carriers in the crystal are negative electrons. An ''electron acceptor'' dopant is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photovoltaics
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially used for electricity generation and as photosensors. A photovoltaic system employs solar modules, each comprising a number of solar cells, which generate electrical power. PV installations may be ground-mounted, rooftop-mounted, wall-mounted or floating. The mount may be fixed or use a solar tracker to follow the sun across the sky. Photovoltaic technology helps to mitigate climate change because it emits much less carbon dioxide than fossil fuels. Solar PV has specific advantages as an energy source: once installed, its operation generates no pollution and no greenhouse gas emissions, it shows scalability in respect of power needs and silicon has large availability in the Earth's crust, although other materials required in PV system m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-iodide-unit-cell-3D-balls.png)