|
Colloidal Gold
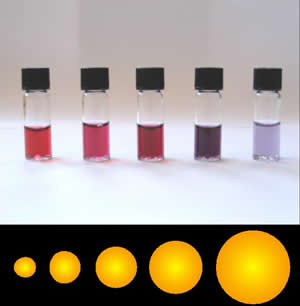
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either wine-red coloured (for spherical particles less than 100 nm) or blue/purple (for larger spherical particles or nanorods). Due to their optical, electronic, and molecular-recognition properties, gold nanoparticles are the subject of substantial research, with many potential or promised applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine. The properties of colloidal gold nanoparticles, and thus their potential applications, depend strongly upon their size and shape. For example, rodlike particles have both a transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly. History Used since ancient times as a method of staining glass colloidal gold was used in the 4th-century Lycurgus Cup, which changes color dependin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gold255
Gold is a chemical element with the symbol Au (from la, aurum) and atomic number 79. This makes it one of the higher atomic number elements that occur naturally. It is a bright, slightly orange-yellow, dense, soft, malleable, and ductile metal in a pure form. Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements and is solid under standard conditions. Gold often occurs in free elemental ( native state), as nuggets or grains, in rocks, veins, and alluvial deposits. It occurs in a solid solution series with the native element silver (as electrum), naturally alloyed with other metals like copper and palladium, and mineral inclusions such as within pyrite. Less commonly, it occurs in minerals as gold compounds, often with tellurium (gold tellurides). Gold is resistant to most acids, though it does dissolve in aqua regia (a mixture of nitric acid and hydrochloric acid), forming a soluble tetrachloroaurate anion. Gold ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stained Glass
Stained glass is coloured glass as a material or works created from it. Throughout its thousand-year history, the term has been applied almost exclusively to the windows of churches and other significant religious buildings. Although traditionally made in flat panels and used as windows, the creations of modern stained glass artists also include three-dimensional structures and sculpture. Modern vernacular usage has often extended the term "stained glass" to include domestic lead light and ''objets d'art'' created from foil glasswork exemplified in the famous lamps of Louis Comfort Tiffany. As a material ''stained glass'' is glass that has been coloured by adding metallic salts during its manufacture, and usually then further decorating it in various ways. The coloured glass is crafted into ''stained glass windows'' in which small pieces of glass are arranged to form patterns or pictures, held together (traditionally) by strips of lead and supported by a rigid frame. Painte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |