|
Collagen Fibers
Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I forms large, eosinophilic fibers known as collagen fibers, which make up most of the rope-like dense connective tissue in the body. Collagen I itself is created by the combination of both a proalpha1 and a proalpha2 chain created by the COL1alpha1 and COL1alpha2 genes respectively. The Col I gene itself takes up a triple-helical conformation due to its Glycine-X-Y structure, x and y being any type of amino acid. Collagen can also be found in two different isoforms, either as a homotrimer or a heterotrimer, both of which can be found during different periods of development. Heterotrimers, in particular, play an important role in wound healing, and are the dominant isoform found in the body. Type I collagen can be found in a myriad of different places in the b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of biomineralization, mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the Gut (anatomy), gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen Helix
In molecular biology, the collagen triple helix or type-2 helix is the main secondary structure of various types of fibrous collagen, including type I collagen. In 1954, Ramachandran & Kartha (13, 14) advanced a structure for the collagen triple helix on the basis of fiber diffraction data. It consists of a triple helix made of the repetitious amino acid sequence glycine-X-Y, where X and Y are frequently proline or hydroxyproline. Collagen folded into a triple helix is known as tropocollagen. Collagen triple helices are often bundled into fibrils which themselves form larger fibres, as in tendons. Structure Glycine, proline, and hydroxyproline must be in their designated positions with the correct configuration. For example, hydroxyproline in the Y position increases the thermal stability of the triple helix, but not when it is located in the X position. The thermal stabilization is also hindered when the hydroxyl group has the wrong configuration. Due to the high abundance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type I, Alpha 2
Collagen alpha-2(I) chain is a protein that in humans is encoded by the ''COL1A2'' gene. This gene encodes one of the chains for type I collagen, the fibrillar collagen found in most connective tissues. Mutations in this gene are associated with osteogenesis imperfecta, Cardiac-valvular and Arthrochlasia type Ehlers-Danlos syndrome, idiopathic osteoporosis, and atypical Marfan syndrome. Symptoms associated with mutations in this gene, however, tend to be less severe than mutations in the gene for alpha-1 type I collagen, since alpha-2 is less abundant. Multiple messages for this gene result from multiple polyadenylation signals, a feature shared by most of the other collagen genes. See also * Type-I collagen * Collagen Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a trip ... Refere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type II Collagen
Type II collagen is the basis for hyaline cartilage, including the articular cartilages at joint surfaces. It is formed by homotrimers of collagen, type II, alpha 1 chains. It makes up 50% of all protein in cartilage and 85–90% of collagen of articular cartilage. Type II collagen is organised into fibrils. This fibrillar network of collagen allows the cartilage to entrap the proteoglycan aggregate, as well as providing tensile strength to the tissue. Oral administration of native type II collagen induces oral tolerance to pathological immune responses and the administration of type II collagen tablets together with paracetamol might be more effective at reducing symptoms of osteoarthritis than paracetamol by itself. See also * Type I collagen Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infantile Cortical Hyperostosis
Infantile cortical hyperostosis (ICH) is a self-limited inflammatory disorder of infants that causes bone changes, soft tissue swelling and irritability. The disease may be present at birth or occur shortly thereafter. The cause is unknown. Both familial and sporadic forms occur. It is also known as Caffey disease or Caffey's disease. Presentation An affected infant typically has the following triad of signs and symptoms: soft-tissue swelling, bone lesions, and irritability. The swelling occurs suddenly, is deep, firm, and may be tender. Lesions are often asymmetric and may affect several parts of the body. Affected bones have included the mandible, tibia, ulna, clavicle, scapula, ribs, humerus, femur, fibula, skull, ilium, and metatarsals. When the mandible (lower jaw bone) is affected, infants may refuse to eat, leading to failure to thrive. Genetics ICH is associated with autosomal dominant pathogenic variants in '' COL1A1'' and possibly '' IFITM5''. Pathophysiolo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type I, Alpha 1
Collagen, type I, alpha 1, also known as alpha-1 type I collagen, is a protein that in humans is encoded by the gene. ''COL1A1'' encodes the major component of type I collagen, the fibrillar collagen found in most connective tissues, including cartilage. Function Collagen is a protein that strengthens and supports many tissues in the body, including cartilage, bone, tendon, skin and the white part of the eye (sclera). The gene produces a component of type I collagen, called the pro-alpha1(I) chain. This chain combines with another pro-alpha1(I) chain and also with a pro-alpha2(I) chain (produced by the gene) to make a molecule of type I procollagen. These triple-stranded, rope-like procollagen molecules must be processed by enzymes outside the cell. Once these molecules are processed, they arrange themselves into long, thin fibrils that cross-link to one another in the spaces around cells. The cross-links result in the formation of very strong mature type I collagen fibers ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osteogenesis Imperfecta
Osteogenesis imperfecta (; OI), colloquially known as brittle bone disease, is a group of genetic disorders that all result in bones that bone fracture, break easily. The range of symptoms—on the skeleton as well as on the body's other Organ (biology), organs—may be mild to severe. Symptoms found in various types of OI include sclera, whites of the eye (sclerae) that are blue instead, short stature, joint hypermobility, loose joints, hearing loss, breathing problems and problems with the teeth (dentinogenesis imperfecta). Potentially life-threatening Complication (medicine), complications, all of which become more common in more severe OI, include: tearing (Dissection (medical), dissection) of the major arteries, such as Aortic dissection, the aorta; pulmonary insufficiency, pulmonary valve insufficiency secondary to distortion of the ribcage; and basilar invagination. The underlying mechanism is usually a problem with connective tissue due to a lack of, or poorly forme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At the lowest range, metal particles smaller than 1 nm are usually called atom clusters instead. Nanoparticles are distinguished from microparticles (1-1000 μm), "fine particles" (sized between 100 and 2500 nm), and "coarse particles" (ranging from 2500 to 10,000 nm), because their smaller size drives very different physical or chemical properties, like colloidal properties and ultrafast optical effects or electric properties. Being more subject to the Brownian motion, they usually do not sediment, like colloid, colloidal particles that conversely are usually understood to range from 1 to 1000 nm. Being much smaller than the wavelengths of visible light (400-700 nm), nanoparticles cannot be seen with ordinary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained the amino a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
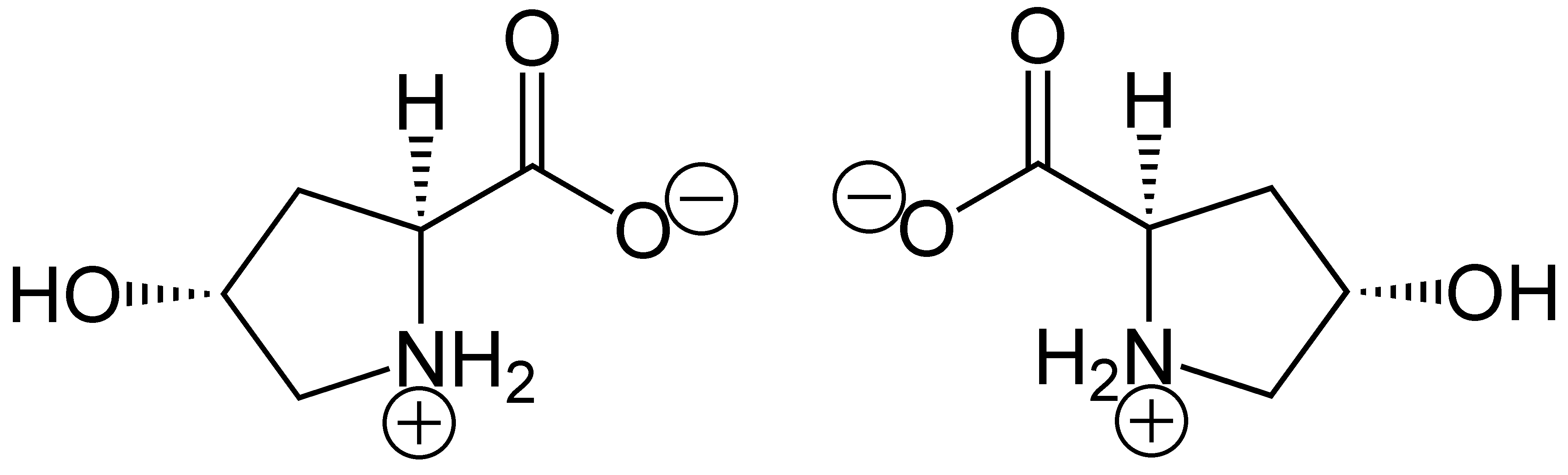
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl 4-hydroxylase following protein synthesis (as a post-translational modification). The enzyme-catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen Type 1
Collagen () is the main structural protein in the extracellular matrix of the connective tissues of many animals. It is the most abundant protein in mammals, making up 25% to 35% of protein content. Amino acids are bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in cartilage, bones, tendons, ligaments, and skin. Vitamin C is vital for collagen synthesis. Depending on the degree of mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes 1% to 2% of muscle tissue and 6% by weight of skeletal muscle. The fibroblast is the most common cell creating collagen in animals. Gelatin, which is used in food and industry, is collagen that was irreversibly hydrolyze ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |