|
Coenzyme Q – Cytochrome C Reductase
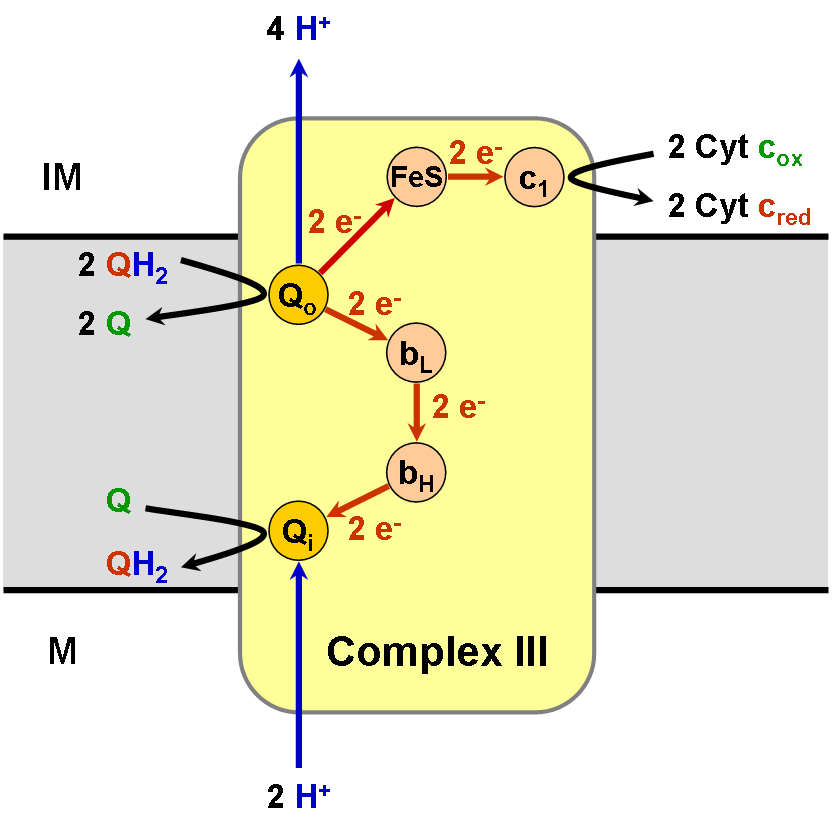
The coenzyme Q : cytochrome ''c'' – oxidoreductase, sometimes called the cytochrome ''bc''1 complex, and at other times complex III, is the third complex in the electron transport chain (), playing a critical role in biochemical generation of ATP (oxidative phosphorylation). Complex III is a multisubunit transmembrane protein encoded by both the mitochondrial (cytochrome b) and the nuclear genomes (all other subunits). Complex III is present in the mitochondria of all animals and all aerobic eukaryotes and the inner membranes of most eubacteria. Mutations in Complex III cause exercise intolerance as well as multisystem disorders. The bc1 complex contains 11 subunits, 3 respiratory subunits (cytochrome B, cytochrome C1, Rieske protein), 2 core proteins and 6 low-molecular weight proteins. Ubiquinol—cytochrome-c reductase catalyzes the chemical reaction :QH2 + 2 ferricytochrome c \rightleftharpoons Q + 2 ferrocytochrome c + 2 H+ Thus, the two substrates of this enzyme ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquinone
Coenzyme Q, also known as ubiquinone and marketed as CoQ10, is a coenzyme family that is ubiquitous in animals and most bacteria (hence the name ubiquinone). In humans, the most common form is coenzyme Q10 or ubiquinone-10. It is a 1,4-benzoquinone, where Q refers to the quinone chemical group and 10 refers to the number of isoprenyl chemical subunits in its tail. In natural ubiquinones, the number can be anywhere from 6 to 10. This family of fat-soluble substances, which resemble vitamins, is present in all respiring eukaryotic cells, primarily in the mitochondria. It is a component of the electron transport chain and participates in aerobic cellular respiration, which generates energy in the form of ATP. Ninety-five percent of the human body's energy is generated this way. Organs with the highest energy requirements—such as the heart, liver, and kidney—have the highest CoQ10 concentrations. There are three redox states of CoQ: fully oxidized (ubiquinone), semiquinone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidoreductase
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix. in ...
|
Human
Humans (''Homo sapiens'') are the most abundant and widespread species of primate, characterized by bipedalism and exceptional cognitive skills due to a large and complex brain. This has enabled the development of advanced tools, culture, and language. Humans are highly social and tend to live in complex social structures composed of many cooperating and competing groups, from families and kinship networks to political states. Social interactions between humans have established a wide variety of values, social norms, and rituals, which bolster human society. Its intelligence and its desire to understand and influence the environment and to explain and manipulate phenomena have motivated humanity's development of science, philosophy, mythology, religion, and other fields of study. Although some scientists equate the term ''humans'' with all members of the genus ''Homo'', in common usage, it generally refers to ''Homo sapiens'', the only extant member. Anatomically moder ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-sulfur Cluster
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome C1
Cytochrome C1 (also known as Complex III subunit 4) is a protein encoded by the ''CYC1'' gene. Cytochrome is a heme-containing subunit of the cytochrome b-c1 complex, which accepts electrons from Rieske protein and transfers electrons to cytochrome c in the mitochondrial respiratory chain. It is formed in the cytosol and targeted to the mitochondrial intermembrane space. Cytochrome c1 belongs to the cytochrome c family of proteins. Function Cytochrome C1 plays a role in the electron transfer during oxidative phosphorylation. As an iron-sulfur protein approaches the b-c1 complex, it accepts an electron from the cytochrome b subunit, then undergoes a conformational change to attach to cytochrome c1. There, the electron carried by the iron-sulfur protein is transferred to the heme carried by cytochrome c1. This electron is then transferred to a heme carried by cytochrome c. This creates a reduced species of cytochrome c, which separates from the b-c1 complex and moves to the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme, or haem (pronounced / hi:m/ ), is a precursor to hemoglobin, which is necessary to bind oxygen in the bloodstream. Heme is biosynthesized in both the bone marrow and the liver. In biochemical terms, heme is a coordination complex "consisting of an iron ion coordinated to a porphyrin acting as a tetradentate ligand, and to one or two axial ligands." The definition is loose, and many depictions omit the axial ligands. Among the metalloporphyrins deployed by metalloproteins as prosthetic groups, heme is one of the most widely used and defines a family of proteins known as hemoproteins. Hemes are most commonly recognized as components of hemoglobin, the red pigment in blood, but are also found in a number of other biologically important hemoproteins such as myoglobin, cytochromes, catalases, heme peroxidase, and endothelial nitric oxide synthase. The word ''haem'' is derived from Greek ''haima'' meaning "blood". Function Hemoproteins have diverse biological functions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prosthetic Group
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein. Not to be confused with the cofactor that binds to the enzyme apoenzyme (either a holoprotein or heteroprotein) by non-covalent binding a non-protein (non-amino acid) This is a component of a conjugated protein that is required for the protein's biological activity. The prosthetic group may be organic (such as a vitamin, sugar, RNA, phosphate or lipid) or inorganic (such as a metal ion). Prosthetic groups are bound tightly to proteins and may even be attached through a covalent bond. They often play an important role in enzyme catalysis. A protein without its prosthetic group is called an apoprotein, while a protein combined with its prosthetic group is called a holoprotein. A non-covalently bound prosthetic group cannot generally be removed from the holoprotein without denaturating the protein. Thus, the term ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Transfer Chain
An electron transport chain (ETC) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H+ ions) across a membrane. The electrons that transferred from NADH and FADH2 to the ETC involves 4 multi-subunit large enzymes complexes and 2 mobile electron carriers. Many of the enzymes in the electron transport chain are membrane-bound. The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions creates an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen as the final electron acceptor. In anaerobic respiration, other electron acceptors are used, such as sulfate. In an electron transport chain, the redox r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytochrome Bc1 Complex
Cytochromes are redox-active proteins containing a heme, with a central Fe atom at its core, as a cofactor. They are involved in electron transport chain and redox catalysis. They are classified according to the type of heme and its mode of binding. Four varieties are recognized by the International Union of Biochemistry and Molecular Biology (IUBMB), cytochromes a, cytochromes b, cytochromes c and cytochrome d. Cytochrome function is linked to the reversible redox change from ferrous (Fe(II)) to the ferric (Fe(III)) oxidation state of the iron found in the heme core. In addition to the classification by the IUBMB into four cytochrome classes, several additional classifications such as cytochrome o and cytochrome P450 can be found in biochemical literature. History Cytochromes were initially described in 1884 by Charles Alexander MacMunn as respiratory pigments (myohematin or histohematin). In the 1920s, Keilin rediscovered these respiratory pigments and named them the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Enzymes
This article lists enzymes by their classification in the International Union of Biochemistry and Molecular Biology's Enzyme Commission (EC) numbering system. * List of EC numbers (EC 5) * List of EC numbers (EC 6) :Oxidoreductases (EC 1) (Oxidoreductase) *Dehydrogenase * Luciferase *DMSO reductase :EC 1.1 (act on the CH-OH group of donors) * :EC 1.1.1 (with NAD+ or NADP+ as acceptor) ** Alcohol dehydrogenase (NAD) ** Alcohol dehydrogenase (NADP) **Homoserine dehydrogenase ** Aminopropanol oxidoreductase **Diacetyl reductase **Glycerol dehydrogenase **Propanediol-phosphate dehydrogenase ** glycerol-3-phosphate dehydrogenase (NAD+) ** D-xylulose reductase **L-xylulose reductase **Lactate dehydrogenase **Malate dehydrogenase **Isocitrate dehydrogenase ** HMG-CoA reductase * :EC 1.1.2 (with a cytochrome as acceptor) * :EC 1.1.3 (with oxygen as acceptor) **Glucose oxidase **L-gulonolactone oxidase **Thiamine oxidase **Xanthine oxidase * :EC 1.1.4 (with a disul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rieske Protein
Rieske proteins are iron–sulfur protein (ISP) components of cytochrome ''bc''1 complexes and cytochrome b6f complexes and are responsible for electron transfer in some biological systems. John S. Rieske and co-workers first discovered the protein and in 1964 isolated an acetylated form of the bovine mitochondrial protein. In 1979 Trumpower's lab isolated the "oxidation factor" from bovine mitochondria and showed it was a reconstitutively-active form of the Rieske iron-sulfur protein It is a unique Fe-2Scluster in that one of the two Fe atoms is coordinated by two histidine residues rather than two cysteine residues. They have since been found in plants, animals, and bacteria with widely ranging electron reduction potentials from -150 to +400 mV. Biological function Ubiquinol-cytochrome-c reductase (also known as bc1 complex or complex III) is an enzyme complex of bacterial and mitochondrial oxidative phosphorylation systems. It catalyses the oxidation-reduction reaction ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferredoxin
Ferredoxins (from Latin ''ferrum'': iron + redox, often abbreviated "fd") are iron–sulfur proteins that mediate electron transfer in a range of metabolic reactions. The term "ferredoxin" was coined by D.C. Wharton of the DuPont Co. and applied to the "iron protein" first purified in 1962 by Mortenson, Valentine, and Carnahan from the anaerobic bacterium '' Clostridium pasteurianum''. Another redox protein, isolated from spinach chloroplasts, was termed "chloroplast ferredoxin". The chloroplast ferredoxin is involved in both cyclic and non-cyclic photophosphorylation reactions of photosynthesis. In non-cyclic photophosphorylation, ferredoxin is the last electron acceptor thus reducing the enzyme NADP+ reductase. It accepts electrons produced from sunlight- excited chlorophyll and transfers them to the enzyme ferredoxin: NADP+ oxidoreductase . Ferredoxins are small proteins containing iron and sulfur atoms organized as iron–sulfur clusters. These biological " capacitors" can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |