|
Chlorophyllide
Chlorophyllide ''a'' and Chlorophyllide ''b'' are the biosynthetic precursors of chlorophyll ''a'' and chlorophyll ''b'' respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide ''a'' is also an intermediate in the biosynthesis of bacteriochlorophylls. Structures Chlorophyllide ''a'', is a carboxylic acid (R=H). In chlorophyllide ''b'', the methyl group at position 13 ( IUPAC numbering for chlorophyllide ''a'') and highlighted in the green box, is replaced with a formyl group. Biosynthesis steps up to formation of protoporphyrin IX In the early steps of the biosynthesis, which starts from glutamic acid, a tetrapyrrole is created by the enzymes deaminase and cosynthetase which transform aminolevulinic acid via porphobilinogen and hydroxymethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to absorb energy from light. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817. The presence of magnesium in chlorophyll was discovered in 1906, and was that element's first detection in living tissue. After initial work done by German chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll Synthase
In enzymology, chlorophyll synthase () is an enzyme that catalyzes the chemical reaction :chlorophyllide a + phytyl diphosphate \rightleftharpoons chlorophyll a + diphosphate The two substrates of this enzyme are chlorophyllide ''a'' and phytyl diphosphate; its two products are chlorophyll ''a'' and diphosphate. The same enzyme can act on chlorophyllide ''b'' to form chlorophyll ''b''. Chlorophyllide a.svg, Chlorophyllide ''a'' Chlorophyll a structure.svg, Chlorophyll ''a'' This enzyme belongs to the family of transferases, specifically those transferring aryl or alkyl groups other than methyl groups. The systematic name of this enzyme class is chlorophyllide-a:phytyl-diphosphate phytyltransferase. This reaction is the final step of the complete biosynthetic pathway to chlorophylls from glutamic acid Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll B
} Chlorophyll ''b'' is a form of chlorophyll. Chlorophyll ''b'' helps in photosynthesis by absorbing light energy. It is more soluble than chlorophyll ''a'' in polar solvents because of its carbonyl group. Its color is green, and it primarily absorbs blue light. In land plants, the light-harvesting antennae around photosystem II contain the majority of chlorophyll ''b''. Hence, in shade-adapted chloroplasts, which have an increased ratio of photosystem II to photosystem I, there is a higher ratio of chlorophyll ''b'' to chlorophyll ''a''. This is adaptive, as increasing chlorophyll ''b'' increases the range of wavelengths absorbed by the shade chloroplasts. Biosynthesis The Chlorophyll ''b'' biosynthetic pathway utilizes a variety of enzymes. In most plants, chlorophyll is derived from glutamate and is synthesised along a branched pathway that is shared with heme and siroheme. The initial steps incorporate glutamic acid into 5-aminolevulinic acid (ALA); two molecules of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aminolevulinic Acid
δ-Aminolevulinic acid (also dALA, δ-ALA, 5ALA or 5-aminolevulinic acid), an endogenous non-proteinogenic amino acid, is the first compound in the porphyrin synthesis pathway, the pathway that leads to heme in mammals, as well as chlorophyll in plants. 5ALA is used in photodynamic detection and surgery of cancer.Wagnières, G.., Jichlinski, P., Lange, N., Kucera, P., Van den Bergh, H. (2014). Detection of Bladder Cancer by Fluorescence Cystoscopy: From Bench to Bedside - the Hexvix Story. Handbook of Photomedicine, 411-426. Medical uses As a precursor of a photosensitizer, 5ALA is also used as an add-on agent for photodynamic therapy. In contrast to larger photosensitizer molecules, it is predicted by computer simulations to be able to penetrate tumor cell membranes. Cancer diagnosis Photodynamic detection is the use of photosensitive drugs with a light source of the right wavelength for the detection of cancer, using fluorescence of the drug. 5ALA, or derivatives thereof, can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacteriochlorophylls
Bacteriochlorophylls (BChl) are photosynthetic pigments that occur in various phototrophic bacterium, bacteria. They were discovered by C. B. van Niel in 1932. They are related to chlorophylls, which are the primary pigments in plants, algae, and cyanobacteria. Organisms that contain bacteriochlorophyll conduct photosynthesis to sustain their energy requirements, but do not produce oxygen as a byproduct. They use wavelengths of light not absorbed by plants or cyanobacteria. Replacement of with protons gives bacteriophaeophytin (BPh), the phaeophytin form. BacterioChlorophyll a.svg, bacteriochlorophyll ''a'' BacterioChlorophyll b.svg, bacteriochlorophyll ''b'' BacterioChlorophyll c.svg, bacteriochlorophyll ''c'' BacterioChlorophyll d.svg, bacteriochlorophyll ''d'' BacterioChlorophyll e.svg, bacteriochlorophyll ''e'' Bacteriochlorophyll f.svg, bacteriochlorophyll ''f'' BacterioChlorophyll g.svg, bacteriochlorophyll ''g'' Structure Bacteriochlorophylls ''a'', ''b'', and ''g' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxymethylbilane
Hydroxymethylbilane, also known as preuroporphyrinogen, is an organic compound that occurs in living organisms during the synthesis of porphyrins, a group of critical substances that include haemoglobin, myoglobin, and chlorophyll. The name is often abbreviated as HMB. The compound is a substituted bilane, a chain of four pyrrole rings interconnected by methylene bridges . The chain starts with a hydroxymethyl group and ends with an hydrogen, in place of the respective methylene bridges. The other two carbon atoms of each pyrrole cycle are connected to an acetic acid group and a propionic acid group , in that order. The compound is generated from four molecules of porphobilinogen by the enzyme porphobilinogen deaminase: The enzyme uroporphyrinogen III synthase closes the chain to form a porphyrinogen a class of compounds with the hexahydroporphine macrocycle; specifically, uroporphyrinogen III. In the absence of the enzyme, the compound undergoes spontaneous cyclization a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Porphobilinogen Deaminase
Porphobilinogen deaminase (hydroxymethylbilane synthase, or uroporphyrinogen I synthase) is an enzyme () that in humans is encoded by the HMBS gene. Porphobilinogen deaminase is involved in the third step of the heme biosynthetic pathway. It catalyzes the head to tail condensation of four porphobilinogen molecules into the linear hydroxymethylbilane while releasing four ammonia molecules: :4 porphobilinogen + H2O \rightleftharpoons hydroxymethylbilane + 4 NH3 Structure and function Functionally, porphobilinogen deaminase catalyzes the loss of ammonia from the porphobilinogen monomer (deamination) and its subsequent polymerization to a linear tetrapyrrole, which is released as hydroxymethylbilane: The structure of 40-42 kDa porphobilinogen deaminase, which is highly conserved amongst organisms, consists of three domains. Domains 1 and 2 are structurally very similar: each consisting of five beta-sheets and three alpha helices in humans. Domain 3 is positioned between the other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III Synthase
Uroporphyrinogen III synthase () is an enzyme involved in the metabolism of the cyclic tetrapyrrole compound porphyrin. It is involved in the conversion of hydroxymethyl bilane into uroporphyrinogen III. This enzyme catalyses the inversion of the final pyrrole unit (ring D) of the linear tetrapyrrole molecule, linking it to the first pyrrole unit (ring A), thereby generating a large macrocyclic structure, uroporphyrinogen III. The enzyme folds into two alpha/beta domains connected by a beta-ladder, the active site being located between the two domains. Pathology A deficiency is associated with Gunther's disease, also known as congenital erythropoietic porphyria (CEP). This is an autosomal recessive In genetics, dominance is the phenomenon of one variant (allele) of a gene on a chromosome masking or overriding the effect of a different variant of the same gene on the other copy of the chromosome. The first variant is termed dominant and t ... inborn error of metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
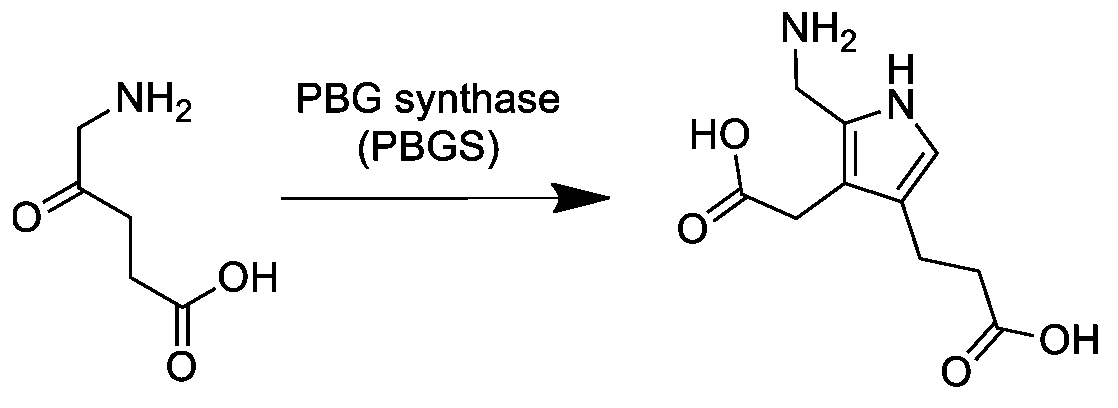
Porphobilinogen
Porphobilinogen (PBG) is an organic compound that occurs in living organisms as an intermediate in the biosynthesis of porphyrins, which include critical substances like hemoglobin and chlorophyll. The structure of the molecule can be described as molecule of pyrrole with sidechains substituted for hydrogen atoms at positions 2, 3 and 4 in the ring (1 being the nitrogen atom); respectively, an aminomethyl group , an acetic acid (carboxymethyl) group , and a propionic acid (carboxyethyl) group . Biosynthesis In the first step of the porphyrin biosynthesis pathway, porphobilinogen is generated from aminolevulinate (ALA) by the enzyme ALA dehydratase. Metabolism In the typical porphyrin biosynthesis pathway, four molecules of porphobilinogen are concatenated by carbons 2 and 5 of the pyrrole ring (adjacent to the nitrogen atom) into hydroxymethyl bilane by the enzyme porphobilinogen deaminase, also known as hydroxymethylbilane synthase. Pathologies Acute intermittent porphyr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biosynthesis
Biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism. The prerequisite elements for biosynthesis include: precursor compounds, chemical energy (e.g. ATP), and catalytic enzymes which may require coenzymes (e.g.NADH, NADPH). These elements create monomers, the building blocks for macromolecules. Some important biological macromolecules include: proteins, which are composed of amino acid monomers joined via peptide bon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uroporphyrinogen III
Uroporphyrinogen III is a tetrapyrrole, the first macrocyclic intermediate in the biosynthesis of heme, chlorophyll, vitamin B12, and siroheme. It is a colorless compound, like other porphyrinogens. Structure The molecular structure of uroporphyrinogen III can be described as a hexahydroporphine core, where each pyrrole ring has the hydrogen atoms on its two outermost carbons replaced by an acetic acid group (, "A") and a propionic acid group (, "P"). The groups are attached in an asymmetric way: going around the macrocycle, the order is AP-AP-AP-PA. Biosynthesis and metabolism In the general porphyrin biosynthesis pathway, uroporphyrinogen III is derived from the linear tetrapyrrole preuroporphyrinogen (a substituted hydroxymethylbilane) by the action of the enzyme uroporphyrinogen-III cosynthase. The conversion entails a reversal of the last pyrrole unit (thus swapping the acetic and propionic acid groups) and a condensation reaction that closes the macrocycle by eliminati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutamic Acid
Glutamic acid (symbol Glu or E; the ionic form is known as glutamate) is an α-amino acid that is used by almost all living beings in the biosynthesis of proteins. It is a non-essential nutrient for humans, meaning that the human body can synthesize enough for its use. It is also the most abundant excitatory neurotransmitter in the vertebrate nervous system. It serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. Its molecular formula is . Glutamic acid exists in three optically isomeric forms; the dextrorotatory -form is usually obtained by hydrolysis of gluten or from the waste waters of beet-sugar manufacture or by fermentation.Webster's Third New International Dictionary of the English Language Unabridged, Third Edition, 1971. Its molecular structure could be idealized as HOOC−CH()−()2−COOH, with two carboxyl groups −COOH and one amino group −. However, in the solid state and mildly acidic water solutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |