|
Chalcogenides
: 220px, Cadmium sulfide, a prototypical metal chalcogenide, is used as a yellow pigment. A chalcogenide is a chemical compound consisting of at least one chalcogen anion and at least one more electropositive element. Although all group 16 elements of the periodic table are defined as chalcogens, the term chalcogenide is more commonly reserved for sulfides, selenides, tellurides, and polonides, rather than oxides. Many metal ores exist as chalcogenides. Photoconductive chalcogenide glasses are used in xerography. Some pigments and catalysts are also based on chalcogenides. The metal dichalcogenide MoS2 is a common solid lubricant. Alkali metal and alkaline earth chalcogenides Alkali metal and alkaline earth monochalcogenides are salt-like, being colourless and often water-soluble. The sulfides tend to undergo hydrolysis to form derivatives containing bisulfide (SH−) anions. The alkali metal chalcogenides often crystallize with the antifluorite structure and the alkaline earth s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcogenide Glass
Chalcogenide glass (pronounced hard ''ch'' as in ''chemistry'') is a glass containing one or more chalcogens (sulfur, selenium and tellurium, but excluding oxygen). Such glasses are covalently bonded materials and may be classified as covalent network solids. Polonium is also a chalcogen but is not used because of its strong radioactivity. Chalcogenide materials behave rather differently from oxides, in particular their lower band gaps contribute to very dissimilar optical and electrical properties. The classical chalcogenide glasses (mainly sulfur-based ones such as As-S or Ge-S) are strong glass-formers and possess glasses within large concentration regions. Glass-forming abilities decrease with increasing molar weight of constituent elements; i.e., S > Se > Te. Chalcogenide compounds such as AgInSbTe and GeSbTe are used in rewritable optical disks and phase-change memory devices. They are fragile glass-formers: by controlling heating and annealing (cooling), they can be swit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chalcogen
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word () principally meaning copper (the term was also used for bronze/brass, any metal in the poetic sense, ore or coin), and the Latinized Greek word , meaning ''born'' or ''produced''. Sulfur has been known since antiquity, and oxygen was recognized as an element in the 18th century. Selenium, tellurium and polonium were discovered in the 19th century, and livermorium in 2000. All of the chalcogens h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 16 Element
The chalcogens (ore forming) ( ) are the chemical elements in group 16 of the periodic table. This group is also known as the oxygen family. Group 16 consists of the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), and the radioactive elements polonium (Po) and livermorium (Lv). Often, oxygen is treated separately from the other chalcogens, sometimes even excluded from the scope of the term "chalcogen" altogether, due to its very different chemical behavior from sulfur, selenium, tellurium, and polonium. The word "chalcogen" is derived from a combination of the Greek word () principally meaning copper (the term was also used for bronze/brass, any metal in the poetic sense, ore or coin), and the Latinized Greek word , meaning ''born'' or ''produced''. Sulfur has been known since antiquity, and oxygen was recognized as an element in the 18th century. Selenium, tellurium and polonium were discovered in the 19th century, and livermorium in 2000. All of the chalcogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenide
A selenide is a chemical compound containing a selenium anion with oxidation number of −2 (Se2−), much as sulfur does in a sulfide. The chemistry of the selenides and sulfides is similar. Similar to sulfide, in aqueous solution, the selenide ion, Se2−, is prevalent only in very basic conditions. In neutral conditions, hydrogen selenide ion, HSe−, is most common. In acid conditions, hydrogen selenide, H2Se, is formed. Some selenides are reactive to oxidation by air. Owing to the greater reducing power of selenide, metal selenides are more easily decomposed to the elements than are sulfides (tellurides are even more labile). Selenides of electropositive metals: such as aluminium selenide readily hydrolyse, even in moist air, evolving toxic hydrogen selenide gas. Pure selenide minerals are rare, instead selenium tends to partially substitute for sulfide in many sulfide minerals. The degree of substitution is only of commercial interest for copper sulfide ores, in which case ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polonide
A polonide is a chemical compound of the radioactive element polonium with any element less electronegative than polonium. Polonides are usually prepared by a direct reaction between the elements at temperatures of around 300–400 °C... They are amongst the most chemically stable compounds of polonium,. and can be divided into two broad groups: *ionic polonides, which appear to contain the Po2− anion; *intermetallic polonides, in which the bonding is more complex. Some polonides are intermediate between these two cases and others are non-stoichiometric compounds. Alloys containing polonium are also classed as polonides. As polonium is immediately below tellurium in the periodic table, there are many chemical and structural similarities between polonides and tellurides. Naturally occurring polonides Lead polonide (PbPo) occurs naturally, as lead is produced in the alpha decay of polonium. Ionic polonides The polonides of the most electropositive metals show classic ionic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
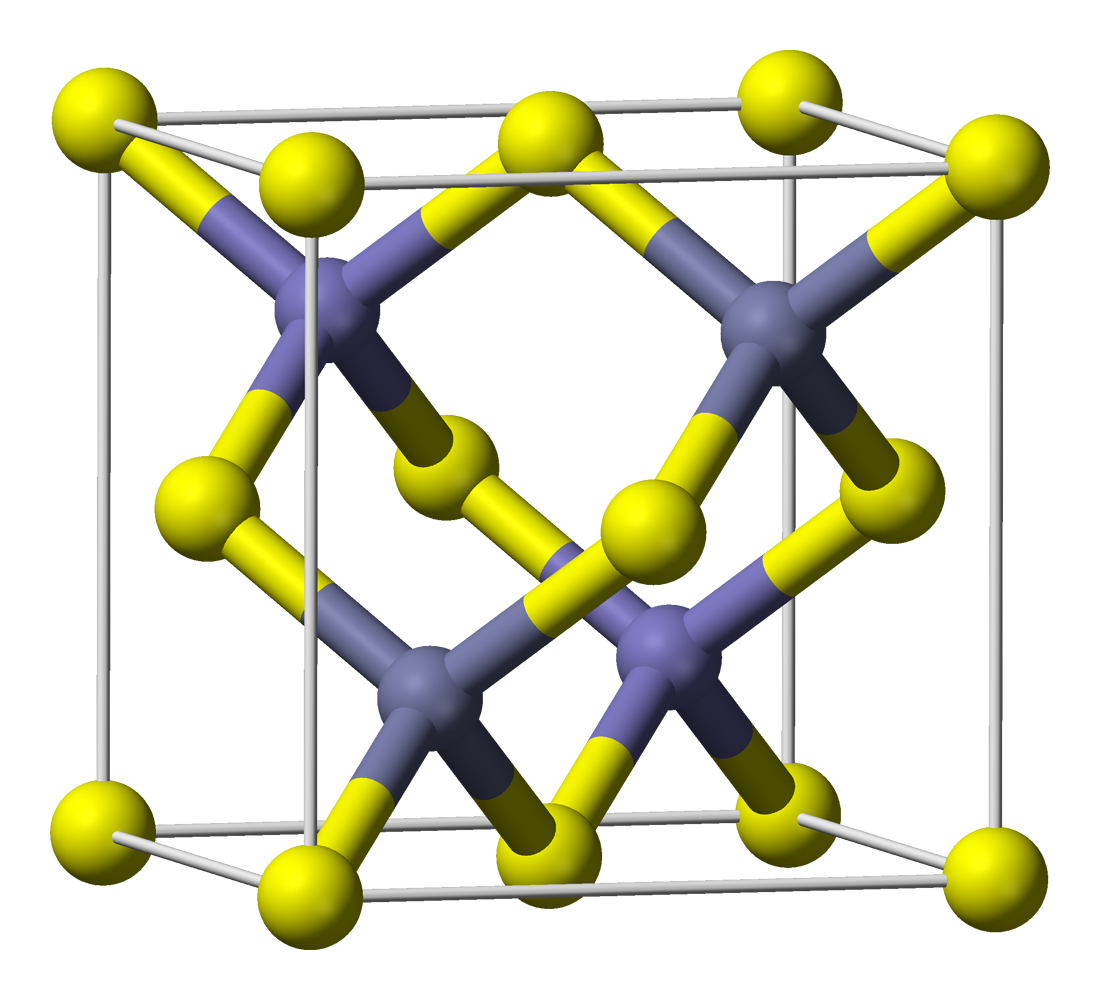
Cadmium Sulfide
Cadmium sulfide is the inorganic compound with the formula CdS. Cadmium sulfide is a yellow solid.Egon Wiberg, Arnold Frederick Holleman (2001''Inorganic Chemistry'' Elsevier It occurs in nature with two different crystal structures as the rare minerals greenockite and hawleyite, but is more prevalent as an impurity substituent in the similarly structured zinc ores sphalerite and wurtzite, which are the major economic sources of cadmium. As a compound that is easy to isolate and purify, it is the principal source of cadmium for all commercial applications. Its vivid yellow color led to its adoption as a pigment for the yellow paint "cadmium yellow" in the 18th century. Production Cadmium sulfide can be prepared by the precipitation from soluble cadmium(II) salts with sulfide ion. This reaction has been used for gravimetric analysis and qualitative inorganic analysis.The preparative route and the subsequent treatment of the product, affects the polymorphic form that is produced ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Sulfide
Zinc sulfide (or zinc sulphide) is an inorganic compound with the chemical formula of ZnS. This is the main form of zinc found in nature, where it mainly occurs as the mineral sphalerite. Although this mineral is usually black because of various impurities, the pure material is white, and it is widely used as a pigment. In its dense synthetic form, zinc sulfide can be transparent, and it is used as a window for visible optics and infrared optics. Structure ZnS exists in two main crystalline forms. This dualism is an example of polymorphism. In each form, the coordination geometry at Zn and S is tetrahedral. The more stable cubic form is known also as zinc blende or sphalerite. The hexagonal form is known as the mineral wurtzite, although it also can be produced synthetically.. The transition from the sphalerite form to the wurtzite form occurs at around 1020 °C. A tetragonal form is also known as the very rare mineral called polhemusite, with the formula . Applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Blende
Sphalerite (sometimes spelled sphaelerite) is a sulfide mineral with the chemical formula . It is the most important ore of zinc. Sphalerite is found in a variety of deposit types, but it is primarily in sedimentary exhalative, Mississippi-Valley type, and volcanogenic massive sulfide deposits. It is found in association with galena, chalcopyrite, pyrite (and other sulfides), calcite, dolomite, quartz, rhodochrosite, and fluorite. German geologist Ernst Friedrich Glocker discovered sphalerite in 1847, naming it based on the Greek word ''sphaleros'', meaning "deceiving", due to the difficulty of identifying the mineral. In addition to zinc, sphalerite is an ore of cadmium, gallium, germanium, and indium. Miners have been known to refer to sphalerite as ''zinc blende'', ''black-jack'', and ''ruby blende''. Marmatite is an opaque black variety with a high iron content. Crystal habit and structure Sphalerite crystallizes in the face-centered cubic zincblende crystal structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diamondoid
In chemistry, diamondoids are variants of the carbon cage molecule known as adamantane (C10H16), the smallest unit cage structure of the diamond crystal lattice. Diamondoids also known as nanodiamonds or condensed adamantanes may include one or more cages (adamantane, diamantane, triamantane, and higher polymantanes) as well as numerous isomeric and structural variants of adamantanes and polymantanes. These diamondoids occur naturally in petroleum deposits and have been extracted and purified into large pure crystals of polymantane molecules having more than a dozen adamantane cages per molecule. These species are of interest as molecular approximations of the diamond cubic framework, terminated with C−H bonds. Cyclohexamantane may be thought of as a nanometer-sized diamond of approximately . Examples Examples include: * Adamantane (C10H16) * Iceane (C12H18) * BC-8 (C14H20) * Diamantane (C14H20) also ''diadamantane'', two face-fused cages * Triamantane (C18H24), also ''triadama ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wurtzite
Wurtzite is a zinc and iron sulfide mineral with the chemical formula , a less frequently encountered Polymorphism (materials science), structural polymorph form of sphalerite. The iron content is variable up to eight percent.Palache, Charles, Harry Berman & Clifford Frondel (1944), ''The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana,'' Yale University 1837-1892, Volume I: Elements, Sulfides, Sulfosalts, Oxides. John Wiley and Sons, Inc., New York. 7th edition, revised and enlarged, pp. 226-228. It is trimorphous with matraite and sphalerite. It occurs in hydrothermal deposits associated with sphalerite, pyrite, chalcopyrite, barite and marcasite. It also occurs in low-temperature clay-ironstone concretions. It was first described in 1861 for an occurrence in the San José Mine, Oruro, Bolivia, Oruro City, Cercado Province (Oruro), Cercado Province, Oruro Department, Bolivia, and named for French chemist Charles-Adolphe Wurtz. It has widespread distribution. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonstoichiometry
In chemistry, non-stoichiometric compounds are chemical compounds, almost always solid inorganic compounds, having elemental composition whose proportions cannot be represented by a ratio of small natural numbers (i.e. an empirical formula); most often, in such materials, some small percentage of atoms are missing or too many atoms are packed into an otherwise perfect lattice work. Contrary to earlier definitions, modern understanding of non-stoichiometric compounds view them as homogeneous, and not mixtures of stoichiometric chemical compounds. Since the solids are overall electrically neutral, the defect is compensated by a change in the charge of other atoms in the solid, either by changing their oxidation state, or by replacing them with atoms of different elements with a different charge. Many metal oxides and sulfides have non-stoichiometric examples; for example, stoichiometric iron(II) oxide, which is rare, has the formula , whereas the more common material is nonstoichi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nickel Arsenide
Nickeline or niccolite is a mineral consisting primarily of nickel arsenide (NiAs). The naturally-occurring mineral contains roughly 43.9% nickel and 56.1% arsenic by mass, but composition of the mineral may vary slightly. Small quantities of sulfur, iron and cobalt are usually present, and sometimes the arsenic is largely replaced by antimony. This last forms an isomorphous series with breithauptite (nickel antimonide). Etymology and history Medieval miners looking for copper in the German Erzgebirge ("Ore Mountains") would sometimes find a red mineral, superficially resembling copper ore. Upon attempting extraction, no copper was produced, and subsequently, the miners would be afflicted with mysterious illness. They blamed a mischievous sprite of German mythology, Nickel (similar to ''Old Nick'') for besetting the copper (German: Kupfer). This German equivalent of "copper-nickel" was used as early as 1694 (other old German synonyms are ''Rotnickelkies'' and ''Arsennickel' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.png)