|
Catenane
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catenane Crystal Structure ChemComm Page634 1991 Commons
In macromolecular chemistry, a catenane () is a mechanically interlocked molecular architecture consisting of two or more interlocked macrocycles, i.e. a molecule containing two or more intertwined rings. The interlocked rings cannot be separated without breaking the covalent bonds of the macrocycles. They are conceptually related to other mechanically interlocked molecular architectures, such as rotaxanes, molecular knots or molecular Borromean rings. Recently the terminology "mechanical bond" has been coined that describes the connection between the macrocycles of a catenane. Catenanes have been synthesised in two different ways: statistical synthesis and template-directed synthesis. Synthesis There are two primary approaches to the organic synthesis of catenanes. The first is to simply perform a ring-closing reaction with the hope that some of the rings will form around other rings giving the desired catenane product. This so-called "statistical approach" led to the f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanically Interlocked Molecular Architectures
In chemistry, mechanically-interlocked molecular architectures (MIMAs) are molecules that are connected as a consequence of their topology. This connection of molecules is analogous to keys on a keychain loop. The keys are not directly connected to the keychain loop but they cannot be separated without breaking the loop. On the molecular level, the interlocked molecules cannot be separated without the breaking of the covalent bonds that comprise the conjoined molecules; this is referred to as a mechanical bond. Examples of mechanically interlocked molecular architectures include catenanes, rotaxanes, molecular knots, and molecular Borromean rings. Work in this area was recognized with the 2016 Nobel Prize in Chemistry to Bernard L. Feringa, Jean-Pierre Sauvage, and J. Fraser Stoddart. The synthesis of such entangled architectures has been made efficient by combining supramolecular chemistry with traditional covalent synthesis, however mechanically interlocked molecular archite ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Borromean Rings
In chemistry, molecular Borromean rings are an example of a mechanically-interlocked molecular architecture in which three macrocycles are interlocked in such a way that breaking any macrocycle allows the others to dissociate. They are the smallest examples of Borromean rings. The synthesis of molecular Borromean rings was reported in 2004 by the group of J. Fraser Stoddart. The so-called Borromeate is made up of three interpenetrated macrocycles formed through templated self assembly as complexes of zinc. The synthesis of the macrocyclic systems involves self-assembles of two organic building blocks: 2,6-diformylpyridine (an aromatic compound with two aldehyde groups positioned '' ortho'' to the nitrogen atom of the pyridine ring) and a symmetric diamine containing a ''meta''-substituted 2,2'-bipyridine group. Zinc acetate is added as the template for the reaction, resulting in one zinc cation in each of the six pentacoordinate complexation sites. Trifluoroacetic acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Switch
A molecular switch is a molecule that can be reversibly shifted between two or more stable states. The molecules may be shifted between the states in response to environmental stimuli, such as changes in pH, light, temperature, an electric current, microenvironment, or in the presence of ions and other ligands. In some cases, a combination of stimuli is required. The oldest forms of synthetic molecular switches are pH indicators, which display distinct colors as a function of pH. Currently synthetic molecular switches are of interest in the field of nanotechnology for application in molecular computers or responsive drug delivery systems. Molecular switches are also important in biology because many biological functions are based on it, for instance allosteric regulation and vision. They are also one of the simplest examples of molecular machines. Biological molecular switches In cellular biology, proteins act as intracellular signaling molecules by activating another protein i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rotaxane
In chemistry, a rotaxane () is a mechanically interlocked molecular architecture consisting of a dumbbell-shaped molecule which is threaded through a macrocycle (see graphical representation). The two components of a rotaxane are kinetically trapped since the ends of the dumbbell (often called ''stoppers'') are larger than the internal diameter of the ring and prevent dissociation (unthreading) of the components since this would require significant distortion of the covalent bonds. Much of the research concerning rotaxanes and other mechanically interlocked molecular architectures, such as catenanes, has been focused on their efficient synthesis or their utilization as artificial molecular machines. However, examples of rotaxane substructure have been found in naturally occurring peptides, including: cystine knot peptides, cyclotides or lasso-peptides such as microcin J25. Synthesis The earliest reported synthesis of a rotaxane in 1967 relied on the statistical probabi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Knots
In chemistry, a molecular knot is a mechanically interlocked molecular architecture that is analogous to a macroscopic knot. Naturally-forming molecular knots are found in organic molecules like DNA, RNA, and proteins. It is not certain that naturally occurring knots are evolutionarily advantageous to nucleic acids or proteins, though knotting is thought to play a role in the structure, stability, and function of knotted biological molecules. The mechanism by which knots naturally form in molecules, and the mechanism by which a molecule is stabilized or improved by knotting, is ambiguous. The study of molecular knots involves the formation and applications of both naturally occurring and chemically synthesized molecular knots. Applying chemical topology and knot theory to molecular knots allows biologists to better understand the structures and synthesis of knotted organic molecules. The term ''knotane'' was coined by Vögtle ''et al.'' in 2000 to describe molecular knots by a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Supramolecular Chemistry
Supramolecular chemistry refers to the branch of chemistry concerning chemical systems composed of a discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from weak intermolecular forces, electrostatic charge, or hydrogen bonding to strong covalent bonding, provided that the electronic coupling strength remains small relative to the energy parameters of the component. While traditional chemistry concentrates on the covalent bond, supramolecular chemistry examines the weaker and reversible non-covalent interactions between molecules. These forces include hydrogen bonding, metal coordination, hydrophobic forces, van der Waals forces, pi–pi interactions and electrostatic effects. Important concepts advanced by supramolecular chemistry include molecular self-assembly, molecular folding, molecular recognition, host–guest chemistry, mechanically-interlocked molecular architectures, and dynamic covalent chemistry. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jeremy Sanders
Jeremy Keith Morris Sanders (born 3 May 1948) is a British chemist and Emeritus Professor in the Department of Chemistry at the University of Cambridge. He is also Editor-in-Chief of Royal Society Open Science. He is known for his contributions to many fields including NMR spectroscopy and supramolecular chemistry. He served as the Pro-Vice-Chancellor for Institutional Affairs at the University of Cambridge, 2011–2015. Education Educated in London at Southmead Primary School and Wandsworth Comprehensive School, he then studied chemistry at Imperial College London where he graduated with a Bachelor of Science degree in 1969 and was awarded the Edmund White Prize. During 1969–72 he carried out his PhD research on lanthanide shift reagents, especially Eu(DPM), the original reagent developed before Eu(FOD) at Churchill College, Cambridge, supervised by Dudley Williams. Career and Research Elected a fellow of Christ's College, Cambridge, in 1972, he spent a postdoctoral y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrathiafulvalene
Tetrathiafulvalene is an organosulfur compound with the formula (. Studies on this heterocyclic compound contributed to the development of molecular electronics. TTF is related to the hydrocarbon fulvalene, , by replacement of four CH groups with sulfur atoms. Over 10,000 scientific publications discuss TTF and its derivatives. Preparation The high level of interest in TTFs has spawned the development of many syntheses of TTF and its analogues. Most preparations entail the coupling of cyclic building blocks such as 1,3-dithiole-2-thion or the related 1,3-dithiole-2-ones. For TTF itself, the synthesis begins with the trithiocarbonate , which is S-methylated and then reduced to give , which is treated as follows: :H2C2S2CH(SCH3) + HBF4 -> 2C2S2CH+F4- + HSCH3 :2 2C2S2CH+F4- + 2 Et3N -> (H2C2S2C)2 + 2 Et3NHBF4 Redox properties Bulk TTF itself has unremarkable electrical properties. Distinctive properties are, however, associated with salts of its oxidized derivatives, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viologen
Viologens are organic compounds with the formula (C5H4NR)2n+. In some viologens, the pyridyl groups are further modified. Viologens are called so, because these compounds produce violet color on reduction [violet + Latin ''gen'', generator of]. The viologen paraquat (R = methyl), is a widely used herbicide. As early as in the 1930s, paraquat was being used as an oxidation-reduction indicator, because it becomes violet on reduction. Other viologens have been commercialized because they can change color reversibly many times through Redox, reduction and oxidation. The name viologen alludes to violet, one color it can exhibit, and the radical ion, radical cation (C5H4NR)2+ is colored intensely blue. Types of viologens As bipyridinium derivatives, the viologens are related to 4,4'-bipyridyl. The basic nitrogen centers in these compounds are alkylated to give viologens: :(C5H4N)2 + 2 RX → [(C5H4NR)2]2+(X−)2 The alkylation is a form of quaternization. When the alkylati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
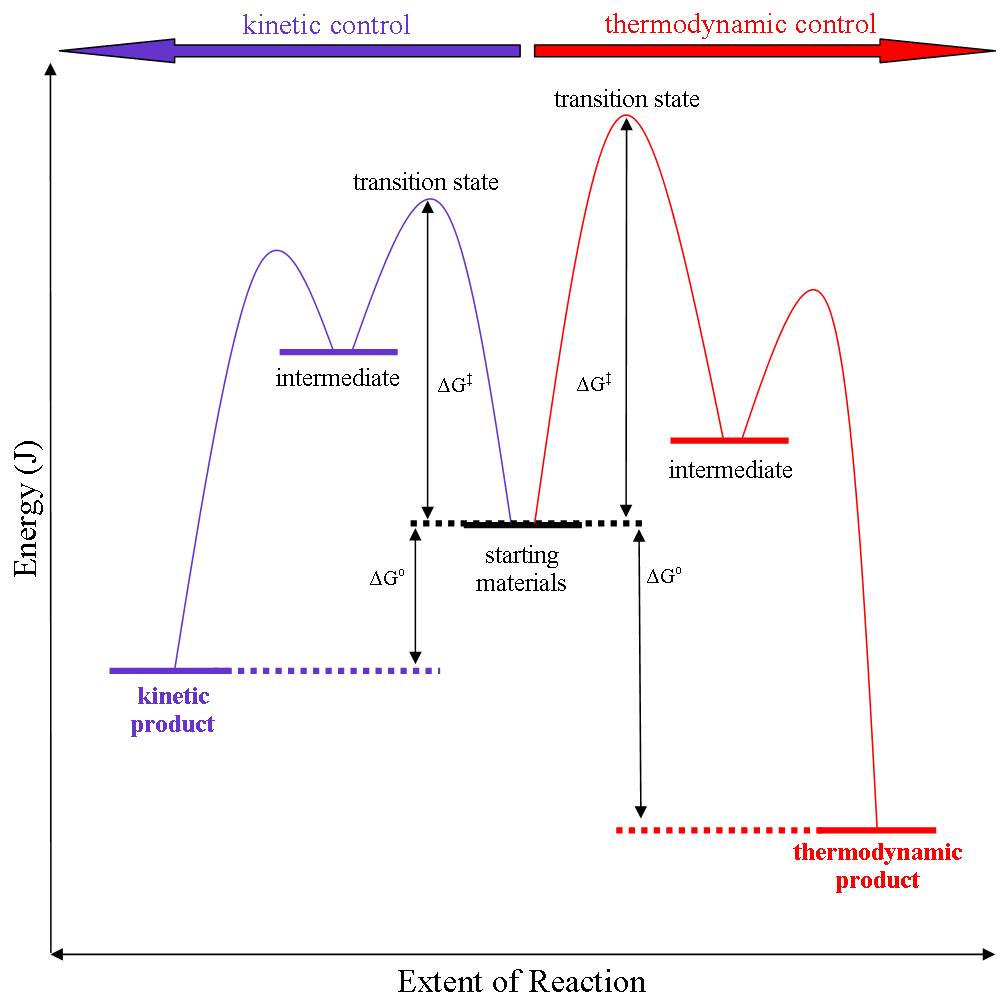
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one pathway having a lower ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |