|
Canine Hepacivirus
''Hepacivirus A'', or Canine hepacivirus (CHV) or Equine hepacivirus (EHV), is a positive-sense single-stranded RNA virus of the genus ''Hepacivirus''. It infects dogs and horses, and causes pulmonary infections in dogs. Unlike the related Hepatitis C virus, it is not known to cause hepatitis in either host. History The virus was isolated in 2011 from a number of dogs suffering from respiratory infections. Later, distinct lineages were isolated from horses in different locations. Genome As of 2012, the genome has not yet been fully sequenced. The available sequence is about 6,500 nucleotides in length. It is predicted to have a polyprotein that can be cleaved into 10 smaller proteins. There is a 'slippery sequence' – A5NNA5 – within the genome which may encode a programmed frameshift. It encodes two envelope proteins (E1 and E2) as well as cysteine and serine proteases. The overall G+C content is 50.7%. Evolution The virus appears to have evolved from the Hepatitis C viru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positive-sense Single-stranded RNA Virus
Positive-strand RNA viruses (+ssRNA viruses) are a group of related viruses that have positive-sense, single-stranded genomes made of ribonucleic acid. The positive-sense genome can act as messenger RNA (mRNA) and can be directly translated into viral proteins by the host cell's ribosomes. Positive-strand RNA viruses encode an RNA-dependent RNA polymerase (RdRp) which is used during replication of the genome to synthesize a negative-sense antigenome that is then used as a template to create a new positive-sense viral genome. Positive-strand RNA viruses are divided between the phyla ''Kitrinoviricota'', ''Lenarviricota'', and ''Pisuviricota'' (specifically classes ''Pisoniviricetes'' and '' Stelpavirictes'') all of which are in the kingdom '' Orthornavirae'' and realm '' Riboviria''. They are monophyletic and descended from a common RNA virus ancestor. In the Baltimore classification system, +ssRNA viruses belong to Group IV. Positive-sense RNA viruses include pathogens s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepacivirus
''Hepacivirus'' is a genus of positive-strand RNA viruses in the family ''Flaviviridae''. The hepatitis C virus (HCV), in species ''Hepacivirus C'', infects humans and is associated with hepatitis and hepatocellular carcinoma. There are fourteen species in the genus which infect a range of other vertebrate. History Hepatitis C virus (HCV), which is the causative agent of hepatitis C in humans, and a member of the species ''Hepacivirus C'', was discovered in 1989. Seven genotypes (1–7) and eighty-six subtypes (1a, 1b etc.) of hepatitis C virus have been named. GBV-B virus (also known as GB virus B) discovered in 1995 is capable of infecting New World monkeys, in particular tamarins. Like HCV it is transmitted by the blood-borne route and similar to HCV it is associated with the viral hepatitis. However GBV-B has never been identified in wild animals and its natural host is not known. Structure Viruses in the genus ''Hepacivirus'' are enveloped and have spherical icosahedral-li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
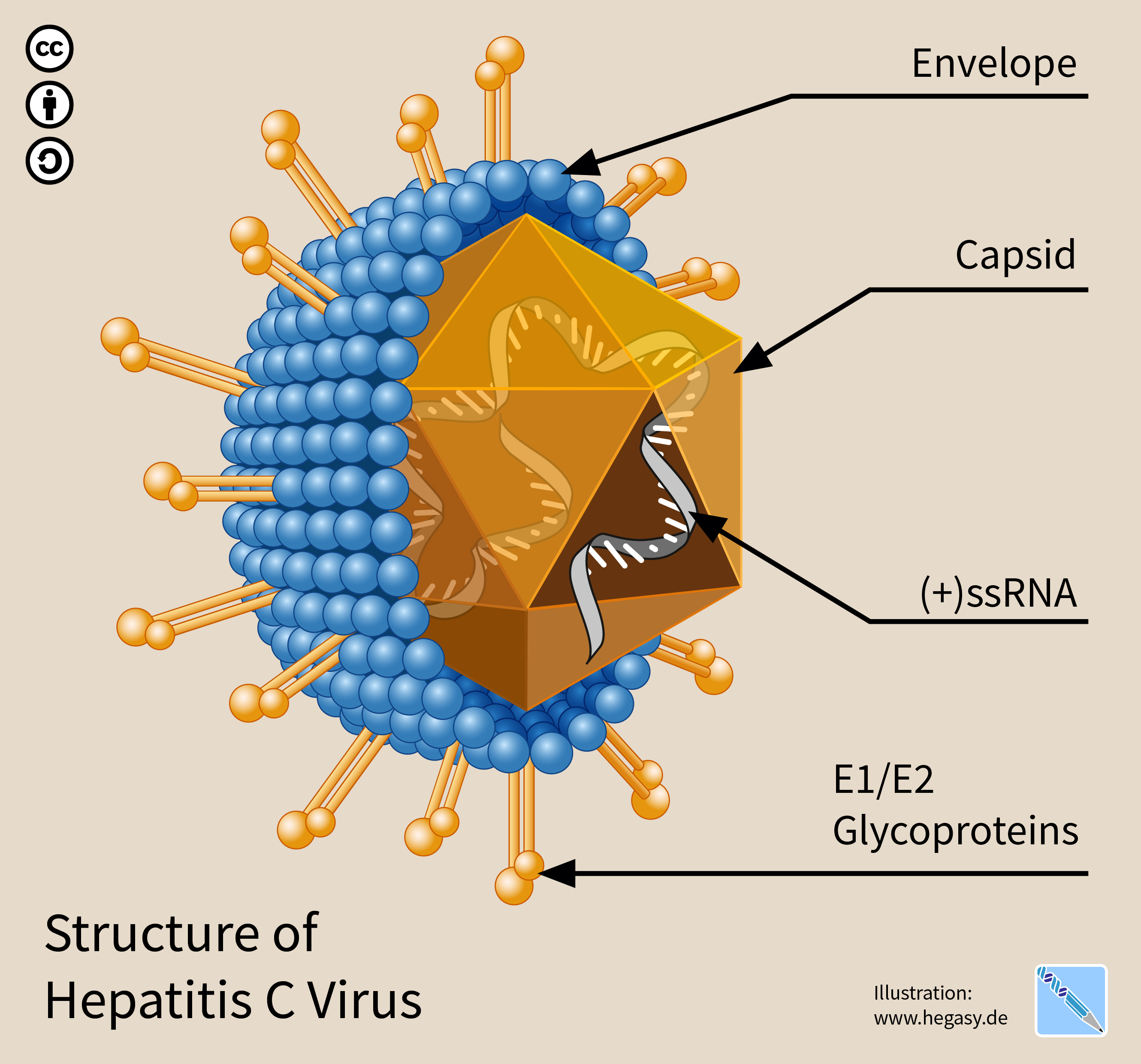
Hepatitis C Virus
The hepatitis C virus (HCV) is a small (55–65 nm in size), enveloped, positive-sense single-stranded RNA virus of the family ''Flaviviridae''. The hepatitis C virus is the cause of hepatitis C and some cancers such as liver cancer ( hepatocellular carcinoma, abbreviated HCC) and lymphomas in humans. Taxonomy The hepatitis C virus belongs to the genus ''Hepacivirus'', a member of the family ''Flaviviridae''. Before 2011, it was considered to be the only member of this genus. However a member of this genus has been discovered in dogs: canine hepacivirus. There is also at least one virus in this genus that infects horses. Several additional viruses in the genus have been described in bats and rodents. Structure The hepatitis C virus particle consists of a lipid membrane envelope that is 55 to 65 nm in diameter. Two viral envelope glycoproteins, E1 and E2, are embedded in the lipid envelope. They take part in viral attachment and entry into the cell. Within the envel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatitis
Hepatitis is inflammation of the liver tissue. Some people or animals with hepatitis have no symptoms, whereas others develop yellow discoloration of the skin and whites of the eyes (jaundice), poor appetite, vomiting, tiredness, abdominal pain, and diarrhea. Hepatitis is ''acute'' if it resolves within six months, and '' chronic'' if it lasts longer than six months. Acute hepatitis can resolve on its own, progress to chronic hepatitis, or (rarely) result in acute liver failure. Chronic hepatitis may progress to scarring of the liver (cirrhosis), liver failure, and liver cancer. Hepatitis is most commonly caused by the virus ''hepatovirus A'', '' B'', '' C'', '' D'', and '' E''. Other viruses can also cause liver inflammation, including cytomegalovirus, Epstein–Barr virus, and yellow fever virus. Other common causes of hepatitis include heavy alcohol use, certain medications, toxins, other infections, autoimmune diseases, and non-alcoholic steatohepatitis (NASH). Hepa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleotide
Nucleotides are organic molecules consisting of a nucleoside and a phosphate. They serve as monomeric units of the nucleic acid polymers – deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), both of which are essential biomolecules within all life-forms on Earth. Nucleotides are obtained in the diet and are also synthesized from common nutrients by the liver. Nucleotides are composed of three subunit molecules: a nucleobase, a five-carbon sugar (ribose or deoxyribose), and a phosphate group consisting of one to three phosphates. The four nucleobases in DNA are guanine, adenine, cytosine and thymine; in RNA, uracil is used in place of thymine. Nucleotides also play a central role in metabolism at a fundamental, cellular level. They provide chemical energy—in the form of the nucleoside triphosphates, adenosine triphosphate (ATP), guanosine triphosphate (GTP), cytidine triphosphate (CTP) and uridine triphosphate (UTP)—throughout the cell for the many cellular func ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyprotein
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slippery Sequence
A slippery sequence is a small section of codon nucleotide sequences (usually UUUAAAC) that controls the rate and chance of ribosomal frameshifting. A slippery sequence causes a faster ribosomal transfer which in turn can cause the reading ribosome to "slip." This allows a tRNA to shift by 1 base (−1) after it has paired with its anticodon, changing the reading frame. A −1 frameshift triggered by such a sequence is a Programmed −1 Ribosomal Frameshift. It is followed by a spacer region, and an RNA secondary structure. Such sequences are common in virus polyproteins. The frameshift occurs due to wobble pairing. The Gibbs free energy of secondary structures downstream give a hint at how often frameshift happens. Tension on the mRNA molecule also plays a role. A list of slippery sequences found in animal viruses is available from Huang et al. Slippery sequences that cause a 2-base slip (−2 frameshift) have been constructed out of the HIV UUUUUUA sequence. See also *Nuclei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frameshift
Ribosomal frameshifting, also known as translational frameshifting or translational recoding, is a biological phenomenon that occurs during translation that results in the production of multiple, unique proteins from a single mRNA. The process can be programmed by the nucleotide sequence of the mRNA and is sometimes affected by the secondary, 3-dimensional mRNA structure. It has been described mainly in viruses (especially retroviruses), retrotransposons and bacterial insertion elements, and also in some cellular genes. Process overview Proteins are translated by reading tri-nucleotides on the mRNA strand, also known as codons, from one end of the mRNA to the other (from the 5' to the 3' end) starting with the amino acid methionine as the start (initiation) codon AUG. Each codon is translated into a single amino acid. The code itself is considered degenerate, meaning that a particular amino acid can be specified by more than one codons. However, a shift of any number of nucleo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Envelope Protein
A viral envelope is the outermost layer of many types of viruses. It protects the genetic material in their life cycle when traveling between host cells. Not all viruses have envelopes. Numerous human pathogenic viruses in circulation are encased in lipid bilayers, and they infect their target cells by causing the viral envelope and cell membrane to fuse. Although there are effective vaccines against some of these viruses, there is no preventative or curative medicine for the majority of them. In most cases, the known vaccines operate by inducing antibodies that prevent the pathogen from entering cells. This happens in the case of enveloped viruses when the antibodies bind to the viral envelope proteins. The membrane fusion event that triggers viral entrance is caused by the viral fusion protein. Many enveloped viruses only have one protein visible on the surface of the particle, which is required for both mediating adhesion to the cell surface and for the subsequent membrane fusi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the naturally occurring proteinogenic amino acids. Only the L-stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, ''sericum''. Serine's structure was estab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |