|
Calthemite
Calthemite is a secondary deposit, derived from concrete, lime, mortar or other calcareous material outside the cave environment.Smith, G.K. (2016). "Calcite straw stalactites growing from concrete structures", Cave and Karst Science 43(1), 4–10. http://bcra.org.uk/pub/candks/index.html?j=127Smith, G K., (2015). "Calcite Straw Stalactites Growing From Concrete Structures". Proceedings of the 30th 'Australian Speleological Federation' conference, Exmouth, Western Australia, edited by Moulds, T. pp 93 -108 Calthemites grow on or under, man-made structures and mimic the shapes and forms of cave speleothems, such as stalactites, stalagmites, flowstone etc.Hill, C A and Forti, P, (1997). Cave Minerals of the World, Second Edition. untsville, Alabama: National Speleological Society Inc. Calthemite is derived from the Latin ''calx'' (genitive ''calcis'') "lime" + Latin < Greek ''théma'', "deposit" meaning ‘something laid down’, (also Mediaeval Latin ''thema'', "deposit") and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calthemite Straw Stalactite
Calthemite is a secondary deposit, derived from concrete, lime, mortar or other calcareous material outside the cave environment.Smith, G.K. (2016). "Calcite straw stalactites growing from concrete structures", Cave and Karst Science 43(1), 4–10. http://bcra.org.uk/pub/candks/index.html?j=127Smith, G K., (2015). "Calcite Straw Stalactites Growing From Concrete Structures". Proceedings of the 30th 'Australian Speleological Federation' conference, Exmouth, Western Australia, edited by Moulds, T. pp 93 -108 Calthemites grow on or under, man-made structures and mimic the shapes and forms of cave speleothems, such as stalactites, stalagmites, flowstone etc.Hill, C A and Forti, P, (1997). Cave Minerals of the World, Second Edition. untsville, Alabama: National Speleological Society Inc. Calthemite is derived from the Latin ''calx'' (genitive ''calcis'') "lime" + Latin < Greek ''théma'', "deposit" meaning ‘something laid down’, (also Mediaeval Latin ''thema'', "deposit") and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
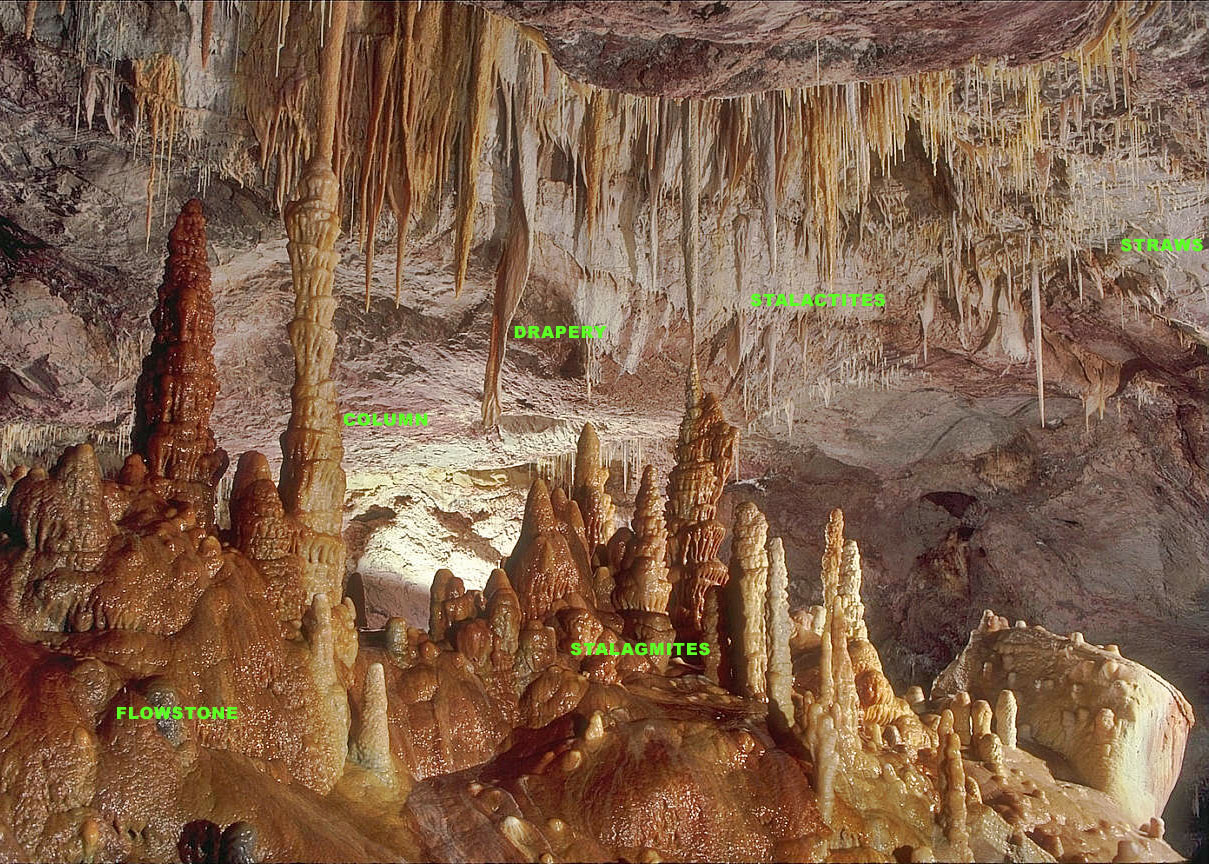
Stalactite
A stalactite (, ; from the Greek 'stalaktos' ('dripping') via ''stalassein'' ('to drip') is a mineral formation that hangs from the ceiling of caves, hot springs, or man-made structures such as bridges and mines. Any material that is soluble and that can be deposited as a colloid, or is in suspension, or is capable of being melted, may form a stalactite. Stalactites may be composed of lava, minerals, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). A stalactite is not necessarily a speleothem, though speleothems are the most common form of stalactite because of the abundance of limestone caves. The corresponding formation on the floor of the cave is known as a stalagmite. Mnemonics have been developed for which word refers to which type of formation; one is that ''stalactite'' has a C for "ceiling", and ''stalagmite'' has a G for "ground". Another example is that ''stalactites'' "hang on ''T''ight" and ''stalagmites'' "''M''ight grow up" � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Speleothem
A speleothem (; ) is a geological formation by mineral deposits that accumulate over time in natural caves. Speleothems most commonly form in calcareous caves due to carbonate dissolution reactions. They can take a variety of forms, depending on their depositional history and environment. Their chemical composition, gradual growth, and preservation in caves make them useful paleoclimatic proxies. Chemical and physical characteristics More than 300 variations of cave mineral deposits have been identified. The vast majority of speleothems are calcareous, composed of calcium carbonate (CaCO3) minerals ( calcite or aragonite). Less commonly, speleothems are made of calcium sulfate (gypsum or mirabilite) or opal. Speleothems of pure calcium carbonate or calcium sulfate are translucent and colorless. The presence of iron oxide or copper provides a reddish brown color. The presence of manganese oxide can create darker colors such as black or dark brown. Speleothems can also be br ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthropocene
The Anthropocene ( ) is a proposed geological epoch dating from the commencement of significant human impact on Earth's geology and ecosystems, including, but not limited to, anthropogenic climate change. , neither the International Commission on Stratigraphy (ICS) nor the International Union of Geological Sciences (IUGS) has officially approved the term as a recognised subdivision of geologic time, although the Anthropocene Working Group (AWG) of the Subcommission on Quaternary Stratigraphy (SQS) of the ICS voted in April 2016 to proceed towards a formal golden spike (GSSP) proposal to define the Anthropocene epoch in the geologic time scale (GTS) and presented the recommendation to the International Geological Congress in August 2016. In May 2019, the AWG voted in favour of submitting a formal proposal to the ICS by 2021, locating potential stratigraphic markers to the mid-twentieth century of the common era. This time period coincides with the start of the Great Accelerati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stalagmite
A stalagmite (, ; from the Greek , from , "dropping, trickling") is a type of rock formation that rises from the floor of a cave due to the accumulation of material deposited on the floor from ceiling drippings. Stalagmites are typically composed of calcium carbonate, but may consist of lava, mud, peat, pitch, sand, sinter, and amberat (crystallized urine of pack rats). The corresponding formation hanging down from the ceiling of a cave is a stalactite. Mnemonics have been developed for which word refers to which type of formation; one is that ''stalactite'' has a C for "ceiling", and ''stalagmite'' has a G for "ground", another is that, as with ants in the pants, the mites go up and the tights (tites) come down. Formation and type Limestone stalagmites The most common stalagmites are speleothems, which usually form in limestone caves. Stalagmite formation occurs only under certain pH conditions within the cavern. They form through deposition of calcium carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime ( calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide ( portlandite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Concrete
Concrete is a composite material composed of fine and coarse aggregate bonded together with a fluid cement (cement paste) that hardens (cures) over time. Concrete is the second-most-used substance in the world after water, and is the most widely used building material. Its usage worldwide, ton for ton, is twice that of steel, wood, plastics, and aluminum combined. Globally, the ready-mix concrete industry, the largest segment of the concrete market, is projected to exceed $600 billion in revenue by 2025. This widespread use results in a number of environmental impacts. Most notably, the production process for cement produces large volumes of greenhouse gas emissions, leading to net 8% of global emissions. Other environmental concerns include widespread illegal sand mining, impacts on the surrounding environment such as increased surface runoff or urban heat island effect, and potential public health implications from toxic ingredients. Significant research and developmen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aragonite
Aragonite is a carbonate mineral, one of the three most common naturally occurring crystal forms of calcium carbonate, (the other forms being the minerals calcite and vaterite). It is formed by biological and physical processes, including precipitation from marine and freshwater environments. The crystal lattice of aragonite differs from that of calcite, resulting in a different crystal shape, an orthorhombic crystal system with acicular crystal. Repeated twinning results in pseudo-hexagonal forms. Aragonite may be columnar or fibrous, occasionally in branching helictitic forms called ''flos-ferri'' ("flowers of iron") from their association with the ores at the Carinthian iron mines. Occurrence The type location for aragonite is Molina de Aragón in the Province of Guadalajara in Castilla-La Mancha, Spain, for which it was named in 1797. Aragonite is found in this locality as cyclic twins inside gypsum and marls of the Keuper facies of the Triassic. This type of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Oxide
Calcium oxide (CaO), commonly known as quicklime or burnt lime, is a widely used chemical compound. It is a white, caustic, alkaline, crystalline solid at room temperature. The broadly used term "'' lime''" connotes calcium-containing inorganic materials, in which carbonates, oxides and hydroxides of calcium, silicon, magnesium, aluminium, and iron predominate. By contrast, ''quicklime'' specifically applies to the single chemical compound calcium oxide. Calcium oxide that survives processing without reacting in building products such as cement is called free lime. Quicklime is relatively inexpensive. Both it and a chemical derivative (calcium hydroxide, of which quicklime is the base anhydride) are important commodity chemicals. Preparation Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate (CaCO3; mineral calcite) in a lime kiln. This is accomplished by heating the material to above ,Merck ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |