|
Bronze Disease (metallurgy)
Bronze disease is an irreversible and nearly inexorable corrosion process that occurs when chlorides come into contact with bronze or other copper-bearing alloys. It can occur as both a dark green coating, or as a much lighter whitish fuzzy or furry green coating. It is not a bacterial infection, but the result of a chemical reaction with the chlorides that usually occurs due to contamination of the bronze object by saltwater or from burial in specific types of soil where chloride salts are present. If not treated, complete destruction of the affected artifact is possible. Treatment is very difficult, costly and not always effective. Transfer of chlorides from the contaminated artefact to other artefacts can spread the condition. Description Bronze disease is the chloride corrosion of cuprous (copper-based) artifacts. It was originally thought to be caused by bacteria. It is contagious in that the chlorides which cause it can spread the condition if they are brought into ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperpigmentation
Hyperpigmentation is the darkening of an area of skin or nails caused by increased melanin. Causes Hyperpigmentation can be caused by sun damage, inflammation, or other skin injuries, including those related to acne vulgaris.James, William; Berger, Timothy; Elston, Dirk (2005). ''Andrews' Diseases of the Skin: Clinical Dermatology''. (10th ed.). Saunders. . People with darker skin tones are more prone to hyperpigmentation, especially with excess sun exposure. Many forms of hyperpigmentation are caused by an excess production of melanin. Hyperpigmentation can be diffuse or focal, affecting such areas as the face and the back of the hands. Melanin is produced by melanocytes at the lower layer of the epidermis. Melanin is a class of pigment responsible for producing color in the body in places such as the eyes, skin, and hair. The process of melanin synthesis (melanogenesis) starts with the oxidation of -tyrosine to by the enzyme tyrosine hydroxylase, then to -dopaquinone and d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brown University
Brown University is a private research university in Providence, Rhode Island. Brown is the seventh-oldest institution of higher education in the United States, founded in 1764 as the College in the English Colony of Rhode Island and Providence Plantations. Brown is one of nine colonial colleges chartered before the American Revolution. Admissions at Brown is among the most selective in the United States. In 2022, the university reported a first year acceptance rate of 5%. It is a member of the Ivy League. Brown was the first college in the United States to codify in its charter that admission and instruction of students was to be equal regardless of their religious affiliation. The university is home to the oldest applied mathematics program in the United States, the oldest engineering program in the Ivy League, and the third-oldest medical program in New England. The university was one of the early doctoral-granting U.S. institutions in the late 19th century, adding masters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bronze
Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such as arsenic or silicon. These additions produce a range of alloys that may be harder than copper alone, or have other useful properties, such as ultimate tensile strength, strength, ductility, or machinability. The three-age system, archaeological period in which bronze was the hardest metal in widespread use is known as the Bronze Age. The beginning of the Bronze Age in western Eurasia and India is conventionally dated to the mid-4th millennium BCE (~3500 BCE), and to the early 2nd millennium BCE in China; elsewhere it gradually spread across regions. The Bronze Age was followed by the Iron Age starting from about 1300 BCE and reaching most of Eurasia by about 500 BCE, although bronze continued to be much more widely used than it is in mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion. In the most common use of the word, this means electrochemical oxidation of metal in reaction with an oxidant such as oxygen, hydrogen or hydroxide. Rusting, the formation of iron oxides, is a well-known example of electrochemical corrosion. This type of damage typically produces oxide(s) or salt(s) of the original metal and results in a distinctive orange colouration. Corrosion can also occur in materials other than metals, such as ceramics or polymers, although in this context, the term "degradation" is more common. Corrosion degrades the useful properties of materials and structures including strength, appearance and permeability to liquids and gases. Many structural ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Canadian Conservation Institute
The Canadian Conservation Institute (CCI; ) is a special operating agency of the federal Department of Canadian Heritage that provides research, information, and services regarding the conservation and preservation of cultural artifacts. Materials and media it handles includes paper, textiles, metals, and glass, as well as electronic media, such as audio tape and compact discs. The CCI offices are located in the Ottawa suburb of Gloucester. Mission The CCI is recognized as a pioneer and leader in the conservation of cultural heritage in Canada. The CCI supports the heritage community in preserving Canada's heritage collections so they can be accessed by current and future generations. The CCI is charged with the duty "to promote the proper care and preservation of Canada's moveable cultural heritage, and to advance the practice, science, and technology of conservation." History The CCI originated within the National Gallery of Canada in 1957, as its Conservation and Scientific R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shellac
Shellac () is a resin secreted by the female lac bug on trees in the forests of India and Thailand. It is processed and sold as dry flakes and dissolved in alcohol to make liquid shellac, which is used as a brush-on colorant, food glaze and wood finish. Shellac functions as a tough natural primer, sanding sealant, tannin-blocker, odour-blocker, stain, and high-gloss varnish. Shellac was once used in electrical applications as it possesses good insulation qualities and it seals out moisture. Phonograph and 78 rpm gramophone records were made of it until they were replaced by vinyl long-playing records from 1948 onwards. From the time it replaced oil and wax finishes in the 19th century, shellac was one of the dominant wood finishes in the western world until it was largely replaced by nitrocellulose lacquer in the 1920s and 1930s. Etymology ''Shellac'' comes from ''shell'' and ''lac'', a calque of French , 'lac in thin pieces', later , 'gum lac'. Most European langua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chelate
Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are called chelants, chelators, chelating agents, or sequestering agents. They are usually organic compounds, but this is not a necessity, as in the case of zinc and its use as a maintenance therapy to prevent the absorption of copper in people with Wilson's disease. Chelation is useful in applications such as providing nutritional supplements, in chelation therapy to remove toxic metals from the body, as contrast agents in MRI scanning, in manufacturing using homogeneous catalysts, in chemical water treatment to assist in the removal of metals, and in fertilizers. Chelate effect The chelate effect is the greater affinity of chelating ligands for a metal ion than that of similar nonchelating (monodentate) ligands for the same metal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethanol
Ethanol (abbr. EtOH; also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is an organic compound. It is an Alcohol (chemistry), alcohol with the chemical formula . Its formula can be also written as or (an ethyl group linked to a hydroxyl group). Ethanol is a Volatility (chemistry), volatile, Combustibility and flammability, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. It is a psychoactive recreational drug, the active ingredient in alcoholic drinks. Ethanol is naturally produced by the fermentation process of Carbohydrate, sugars by yeasts or via Petrochemistry, petrochemical processes such as ethylene hydration. It has medical applications as an antiseptic and disinfectant. It is used as a chemical solvent and in the Chemical synthesis, synthesis of organic compounds, and as a Alcohol fuel, fuel source. Ethanol also can be dehydrated to make ethylene, an important chemical feedstock. As of 2006, world produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
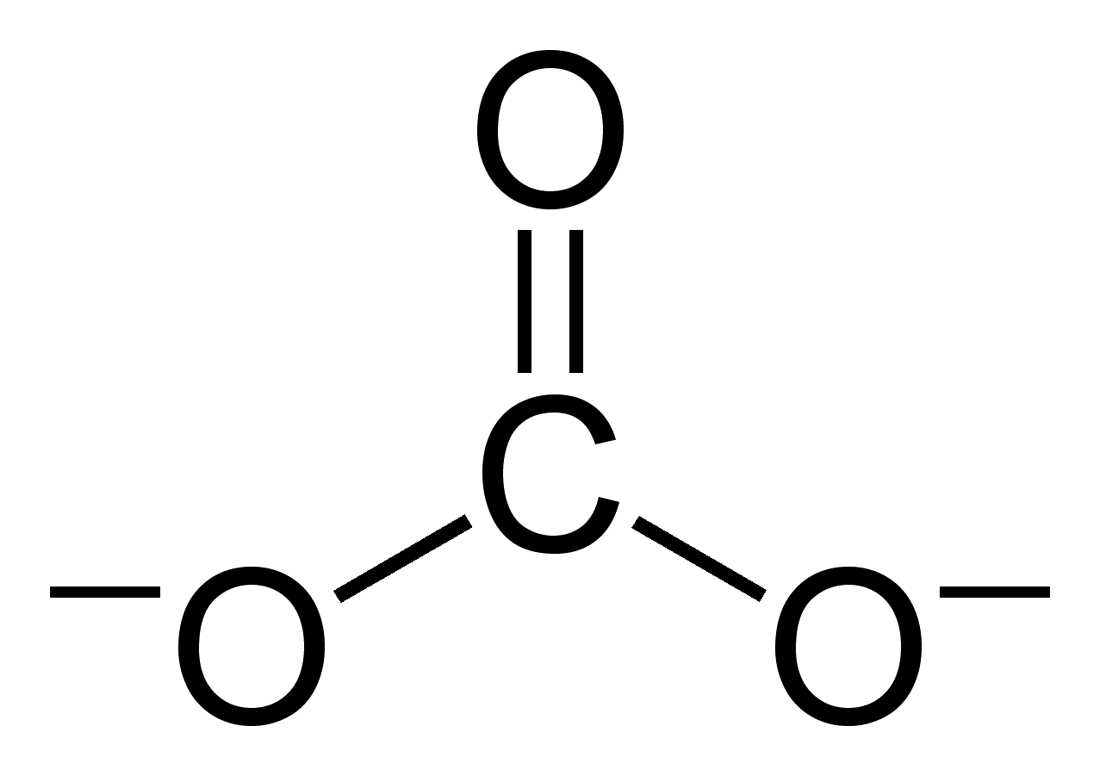
Carbonate Crystals Formed On Ancient Bronze Coin
A carbonate is a salt of carbonic acid (H2CO3), characterized by the presence of the carbonate ion, a polyatomic ion with the formula . The word ''carbonate'' may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O–)2. The term is also used as a verb, to describe carbonation: the process of raising the concentrations of carbonate and bicarbonate ions in water to produce carbonated water and other carbonated beverageseither by the addition of carbon dioxide gas under pressure or by dissolving carbonate or bicarbonate salts into the water. In geology and mineralogy, the term "carbonate" can refer both to carbonate minerals and carbonate rock (which is made of chiefly carbonate minerals), and both are dominated by the carbonate ion, . Carbonate minerals are extremely varied and ubiquitous in chemically precipitated sedimentary rock. The most common are calcite or calcium carbonate, CaCO3, the chief constituent of limestone (as well as t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzotriazole
Benzotriazole (BTA) is a heterocyclic compound with the chemical formula C6H5N3. Its five-membered ring contains three consecutive nitrogen atoms. This bicyclic compound may be viewed as fused rings of the aromatic compounds benzene and triazole. This white-to-light tan solid has a variety of uses, for instance, as a corrosion inhibitor for copper. Structure Benzotriazole features two fused rings. Its five-membered ring can exist in tautomers A and B, and the derivatives of both tautomers, structures C and D, can also be produced: : Various structural analyses with UV, IR and 1H-NMR spectra indicated that isomer A is predominantly present at room temperature. The bond between positions 1 and 2 and the one between positions 2 and 3 have proved to have the same bond properties. Moreover, the proton does not tightly bind to any of the nitrogen atoms, but rather migrates rapidly between positions 1 and 3. Therefore, the BTA can lose a proton to act as a weak acid (pKa = 8.2) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Complex Ion
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |