|
Bmim
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobe, hydrophobic and non-water-soluble ionic liquid with a melting point of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water. Preparation BMIM-PF6 is commercially available. It may be obtained in two steps: BMIM-Cl is synthesized by alkylating 1-methylimidazole with 1-chlorobutane. A metathesis reaction with potassium hexafluorophosphate gives the desired compound; the tetrafluoroborate may be prepared by analogously using potassium tetrafluoroborate. : See also * 1-Butyl-3-methylimidazolium tetrachloroferrate References Further reading * {{DEFAULTSORT:Butyl-3-methylimidazolium hexafluorophosphate, 1- Hexafluorophosphates Imidazolium compounds Ionic liquids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-Butyl-3-methylimidazolium Tetrachloroferrate
1-Butyl-3-methylimidazolium tetrachloroferrate is a magnetic ionic liquid. It can be obtained from 1-butyl-3-methylimidazolium chloride and ferric chloride. It has quite low water solubility. Due to the presence of the high spin FeCl4 anion, the liquid is paramagnetic and a magnetic susceptibility of 40.6 × 10−6 emu g−1 is reported. A simple small neodymium magnet A hard_disk_drive.html"_;"title="Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive">Nickel-plated_neodymium_magnet_on_a_bracket_from_a_hard_disk_drive_ file:Nd-magnet.jpg.html" ;"title="hard_disk_drive_.html" ;"title="hard_disk_d ... suffices to attract the liquid in a test tube. References Magnetism Ionic liquids Imidazolium compounds category:ferrates Iron(III) compounds Iron_complexes Chlorometallates {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hexafluorophosphate
Hexafluorophosphate is an anion with chemical formula of . It is an octahedral species that imparts no color to its salts. is isoelectronic with sulfur hexafluoride, , and the hexafluorosilicate dianion, , and hexafluoroantimonate . In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion. Synthesis Hexafluorophosphate salts can be prepared by the reaction of phosphorus pentachloride and alkali or ammonium halide in a solution of hydrofluoric acid: : Hexafluorophosphoric acid can be prepared by direct reaction of hydrogen fluoride with phosphorus pentafluoride. It is a strong Brønsted acid that is typically generated ''in situ'' immediately before its use. : These reactions require specialized equipment to safely handle the hazards associated with hydrofluoric acid and hydrogen fluoride. Quantitative analysis Several methods of quantitative analysis for the hexafluorophosphate ion have been de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
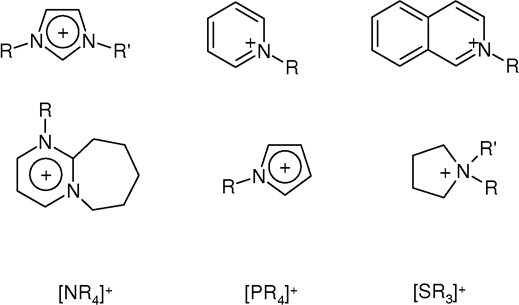
Ionic Liquid
An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below a specific temperature, such as . While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses. Ionic liquids have many potential applications. They are powerful solvents and can be used as electrolytes. Salts that are liquid at near-ambient temperature are important for electric battery applications, and have been considered as sealants due to their very low vapor pressure. Any salt that melts without decomposing or vaporizing usually yields an ionic liquid. Sodium chloride (NaCl), for example, melts at into a liquid that consists largely of sodium cations () and chloride anions (). Conversely, when an ionic liquid is cooled, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrophobe
In chemistry, hydrophobicity is the physical property of a molecule that is seemingly repelled from a mass of water (known as a hydrophobe). In contrast, hydrophiles are attracted to water. Hydrophobic molecules tend to be nonpolar and, thus, prefer other neutral molecules and nonpolar solvents. Because water molecules are polar, hydrophobes do not dissolve well among them. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle. Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds. Hydrophobic is often used interchangeably with lipophilic, "fat-loving". However, the two terms are not synonymous. While hydrophobic substances are usually lipophilic, there are exceptions, suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Green Chem
''Green Chemistry'' is a monthly peer-reviewed scientific journal covering every aspect of sustainable chemistry and its implementation in chemical engineering. It is published by the Royal Society of Chemistry and was established in 1999 by James Clark (University of York). Articles published in this journal are intended to be conceptually accessible to a wide audience. The editors-in-chief is Philip Jessop ( Queen's University). Article types * Research papers (which contain original scientific work that has not been published previously) * Communications (original scientific work that is of an urgent nature and that has not been published previously) * Green Chemistry news (an easy-to-read magazine style section) Abstracting, indexing, and impact factor According to the ''Journal Citation Reports'', the journal has a 2020 impact factor of 10.182. It is indexed in the following bibliographic databases: *Scopus * Web of Science See also *List of chemistry journals This is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1-methylimidazole
1-Methylimidazole or ''N''-methylimidazole is an aromatic heterocyclic organic compound with the formula CH3C3H3N2. It is a colourless liquid that is used as a specialty solvent, a base, and as a precursor to some ionic liquids. It is a fundamental nitrogen heterocycle and as such mimics for various nucleoside bases as well as histidine and histamine. Basicity With the ''N''-methyl group, this particular derivative of imidazole cannot tautomerize. It is slightly more basic than imidazole, as indicated by the pKa's of the conjugate acids of 7.0 and 7.4. Methylation also provides a significantly lower melting point, which makes 1-methylimidazole a useful solvent. Synthesis 1-Methylimidazole is prepared mainly by two routes industrially. The main one is acid-catalysed methylation of imidazole by methanol. The second method involves the Radziszewski reaction from glyoxal, formaldehyde, and a mixture of ammonia and methylamine. :(CHO)2 + CH2O + CH3NH2 + NH3 → H2C2N( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Hexafluorophosphate
Potassium hexafluorophosphate is the chemical compound with the chemical formula, formula KPF6. This colourless salt consists of potassium cations and hexafluorophosphate anions. It is prepared by the reaction: :Phosphorus pentachloride, PCl5 + Potassium chloride, KCl + 6 HF → KPF6 + 6 HCl This exothermic reaction is conducted in liquid hydrogen fluoride. The salt is stable in hot alkaline aqueous solution, from which it can be recrystallized. The sodium and ammonium salts are more soluble in water whereas the rubidium and caesium salts are less so. KPF6 is a common laboratory source of the hexafluorophosphate anion, a non-coordinating anion that confers lipophilicity to its salts. These salts are often less soluble than the closely related tetrafluoroborates. References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Tetrafluoroborate
Potassium is the chemical element with the symbol K (from Neo-Latin ''kalium'') and atomic number19. Potassium is a silvery-white metal that is soft enough to be cut with a knife with little force. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, that is easily removed to create an ion with a positive charge – a cation, that combines with anions to form salts. Potassium in nature occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in sea water (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BMIM
1-Butyl-3-methylimidazolium hexafluorophosphate, also known as BMIM-PF6, is a viscous, colourless, hydrophobe, hydrophobic and non-water-soluble ionic liquid with a melting point of -8 °C. Together with 1-butyl-3-methylimidazolium tetrafluoroborate, BMIM-BF4, it is one of the most widely studied ionic liquids. It is known to very slowly decompose in the presence of water. Preparation BMIM-PF6 is commercially available. It may be obtained in two steps: BMIM-Cl is synthesized by alkylating 1-methylimidazole with 1-chlorobutane. A metathesis reaction with potassium hexafluorophosphate gives the desired compound; the tetrafluoroborate may be prepared by analogously using potassium tetrafluoroborate. : See also * 1-Butyl-3-methylimidazolium tetrachloroferrate References Further reading * {{DEFAULTSORT:Butyl-3-methylimidazolium hexafluorophosphate, 1- Hexafluorophosphates Imidazolium compounds Ionic liquids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analytical And Bioanalytical Chemistry
''Analytical and Bioanalytical Chemistry'' is a peer-reviewed scientific journal publishing research articles in the broad field of analytical and bioanalytical chemistry. Some of the subjects covered are the development of instruments for mass spectrometry, metallomics, ionics, and the analytical characterization of nano- and biomaterials. The journal was first published in 1862 under the name ''Fresenius’ Journal of Analytical Chemistry''. In 2002 the journal was renamed to ''Analytical and Bioanalytical Chemistry''. Impact factor ''Analytical and Bioanalytical Chemistry'' had a 2018 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a given journal, as i ... of 3.286, ranking it 18th out of 84 in the subject category " Chemistry, Analytical" and 21st out of 79 in "Biochemical Research Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |