|
Bioisotere
In medicinal chemistry, bioisosteres are chemical substituents or groups with similar physical or chemical properties which produce broadly similar biological properties to another chemical compound. In drug design, the purpose of exchanging one bioisostere for another is to enhance the desired biological or physical properties of a compound without making significant changes in chemical structure. The main use of this term and its techniques are related to pharmaceutical sciences. Bioisosterism is used to reduce toxicity, change bioavailability, or modify the activity of the lead compound, and may alter the metabolism of the lead. Examples Classical bioisosteres Classical bioisosterism was originally formulated by James Moir and refined by Irving Langmuir as a response to the observation that different atoms with the same valence electron structure had similar biological properties. For example, the replacement of a hydrogen atom with a fluorine atom at a site of metab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Medicinal Chemistry
Medicinal or pharmaceutical chemistry is a scientific discipline at the intersection of chemistry and pharmacy involved with designing and developing pharmaceutical drugs. Medicinal chemistry involves the identification, synthesis and development of new chemical entities suitable for therapeutic use. It also includes the study of existing drugs, their biological properties, and their quantitative structure-activity relationships (QSAR). Medicinal chemistry is a highly interdisciplinary science combining organic chemistry with biochemistry, computational chemistry, pharmacology, molecular biology, statistics, and physical chemistry. Compounds used as medicines are most often organic compounds, which are often divided into the broad classes of small organic molecules (e.g., atorvastatin, fluticasone, clopidogrel) and "biologics" (infliximab, erythropoietin, insulin glargine), the latter of which are most often medicinal preparations of proteins (natural and recombinant ant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl Methylthiophene Replacement
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an enabling disclosure of the invention."A patent is not the grant of a right to make or use or sell. It does not, directly or indirectly, imply any such right. It grants only the right to exclude others. The supposition that a right to make is created by the patent grant is obviously inconsistent with the established distinctions between generic and specific patents, and with the well-known fact that a very considerable portion of the patents granted are in a field covered by a former relatively generic or basic patent, are tributary to such earlier patent, and cannot be practiced unless by license thereunder." – ''Herman v. Youngstown Car Mfg. Co.'', 191 F. 579, 584–85, 112 CCA 185 (6th Cir. 1911) In most countries, patent rights fall under private law and the patent holder mus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Etofenprox
Etofenprox is a pyrethroid derivative which is used as an insecticide. Mitsui Chemicals Agro Inc. is the main manufacturer of the chemical. It is also used as an ingredient in flea medication for cats and dogs. General uses Etofenprox is an insecticide which disturbs insect nervous systems following direct contact or ingestion, and which is active against a broad spectrum of pests. It is used in agriculture, horticulture, viticulture, forestry, animal health and public health against many insect pests, for instance Lepidoptera, Hemiptera, Coleoptera, Diptera, Thysanoptera, and Hymenoptera. In agriculture, etofenprox is used on a broad range of crops such as rice, fruits, vegetables, corn, soybeans, and tea. It is poorly absorbed by roots and little translocation occurs within plants. In the public health sector, etofenprox is used for vector control either by direct application in infested areas or indirectly by impregnating fabrics, such as mosquito nets. Etofenprox is used at l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repellent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrethroid
A pyrethroid is an organic compound similar to the natural pyrethrins, which are produced by the flowers of pyrethrums (''Chrysanthemum cinerariaefolium'' and ''Chrysanthemum coccineum, C. coccineum''). Pyrethroids are used as commercial and household insecticides. In household concentrations pyrethroids are generally harmless to humans. However, pyrethroids are toxic to insects such as bees, dragonflies, mayflies, Horse-fly, gadflies, and some other invertebrates, including those that constitute the base of aquatic and terrestrial food webs. Pyrethroids are toxic to aquatic organisms, especially fish.Pyrethroids fact sheet from the Illinois Department of Public Health. They have been shown to be an effective control measure for malaria outbreaks, through indoor applications. ...
|
Organosilicon
Organosilicon compounds are organometallic compounds containing carbon–silicon bonds. Organosilicon chemistry is the corresponding science of their preparation and properties. Most organosilicon compounds are similar to the ordinary organic compounds, being colourless, flammable, hydrophobic, and stable to air. Silicon carbide is an ''inorganic'' compound. History In 1846 Von Ebelman's had synthesized Tetraethyl orthosilicate (Si(OC2H5)4). In 1863 Friedel and Crafts managed to make the first organosilieon compound with C-Si bonds which gone byound the syntheses of orthosilicic acid esters. The same year they also described a «polysilicic acid ether» in the preparation of ethyl- and methyl-o-silicic acid. The early extensive research in the field of organosilicon compounds was pioneerd in the beginning of 20th century by Frederic Kipping. He also had coined the term «silicone» (akin to ketones) in relation to these materials in 1904. In recognition of Kipping's achiev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silafluofen
Silafluofen is a fluorinated organosilicon pyrethroid insecticide. Silafluofen is used agriculturally against soil-borne insects such as termites, and as a wood preservative. It is registered in Asia (India, Japan, Taiwan, Vietnam) since at least 1995 for crops such as drupes In botany, a drupe (or stone fruit) is an indehiscent fruit in which an outer fleshy part (exocarp, or skin, and mesocarp, or flesh) surrounds a single shell (the ''pit'', ''stone'', or '' pyrena'') of hardened endocarp with a seed (''kernel'') ..., tea and rice, but has not been notified or authorised in the European Union for example. References Pyrethroids Organofluorides Organosilicon compounds Diphenyl ethers {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xanthine
Xanthine ( or ; archaically xanthic acid; systematic name 3,7-dihydropurine-2,6-dione) is a purine base (genetics), base found in most human body tissues and fluids, as well as in other organisms. Several stimulants are derived from xanthine, including caffeine, theophylline, and theobromine. Xanthine is a product on the pathway of purine degradation. * It is created from guanine by guanine deaminase. * It is created from hypoxanthine by xanthine oxidoreductase. * It is also created from xanthosine by purine nucleoside phosphorylase. Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme. Use and manufacturing Xanthine is used as a drug precursor (chemistry), precursor for human and animal medications, and is manufactured as a pesticide ingredient. Clinical significance Derivatives of xanthine (known collectively as xanthines) are a group of alkaloids commonly used for their effects as mild stimulants and as bronchodilators, notably in the t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
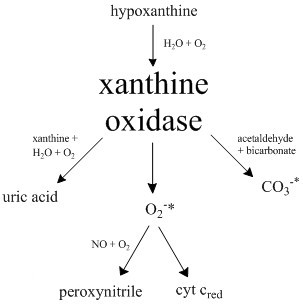
Xanthine Oxidase
Xanthine oxidase (XO, sometimes XAO) is a form of xanthine oxidoreductase, a type of enzyme that generates reactive oxygen species. These enzymes catalyze the oxidation of hypoxanthine to xanthine and can further catalyze the oxidation of xanthine to uric acid. These enzymes play an important role in the catabolism of purines in some species, including humans. Xanthine oxidase is defined as an ''enzyme activity'' (EC 1.17.3.2). The same protein, which in humans has the HGNC approved gene symbol ''XDH'', can also have xanthine dehydrogenase activity (EC 1.17.1.4). Most of the protein in the liver exists in a form with xanthine dehydrogenase activity, but it can be converted to xanthine oxidase by reversible sulfhydryl oxidation or by irreversible proteolytic modification. Reaction The following chemical reactions are catalyzed by xanthine oxidase: * hypoxanthine + H2O + O2 \rightleftharpoons xanthine + H2O2 * xanthine + H2O + O2 \rightleftharpoons uric acid + H2O2 * Xanthine o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alloxanthine
Oxipurinol (INN, or oxypurinol USAN) is an inhibitor of xanthine oxidase. It is an active metabolite of allopurinol and it is cleared renally. In cases of renal disease, this metabolite will accumulate to toxic levels. By inhibiting xanthine oxidase, it reduces uric acid production. High serum uric acid levels may result in gout, kidney stones Kidney stone disease, also known as nephrolithiasis or urolithiasis, is a crystallopathy where a solid piece of material (kidney stone) develops in the urinary tract. Kidney stones typically form in the kidney and leave the body in the urine s ..., and other medical conditions. References Pyrazolopyrimidines Xanthine oxidase inhibitors Human drug metabolites {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |