|
Bioburden
Bioburden is normally defined as the number of bacteria living on a surface that has not been sterilized. The term is most often used in the context of bioburden testing, also known as microbial limit testing, which is performed on pharmaceutical products and medical products for quality control purposes. Products or components used in the pharmaceutical or medical field require control of microbial levels during processing and handling. Bioburden or microbial limit testing on these products proves that these requirements have been met. Bioburden testing for medical devices made or used in the USA is governed by Title 21 of the Code of Federal Regulations and worldwide by ISO 11737. The aim of bioburden testing is to measure the total number of viable micro-organisms (total microbial count) on a medical device prior to its final sterilization before implantation or use. 21 C.F.R. 211.110 (a)(6) states that bioburden in-process testing must be conducted pursuant to written proc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biofouling
Biofouling or biological fouling is the accumulation of microorganisms, plants, algae, or small animals where it is not wanted on surfaces such as ship and submarine hulls, devices such as water inlets, pipework, grates, ponds, and rivers that cause degradation to the primary purpose of that item. Such accumulation is referred to as '' epibiosis'' when the host surface is another organism and the relationship is not parasitic. Since biofouling can occur almost anywhere water is present, biofouling poses risks to a wide variety of objects such as boat hulls and equipment, medical devices and membranes, as well as to entire industries, such as paper manufacturing, food processing, underwater construction, and desalination plants. Anti-fouling is the ability of specifically designed materials (such as toxic biocide paints, or non-toxic paints) to remove or prevent biofouling. The buildup of biofouling on marine vessels poses a significant problem. In some instances, the hull struc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
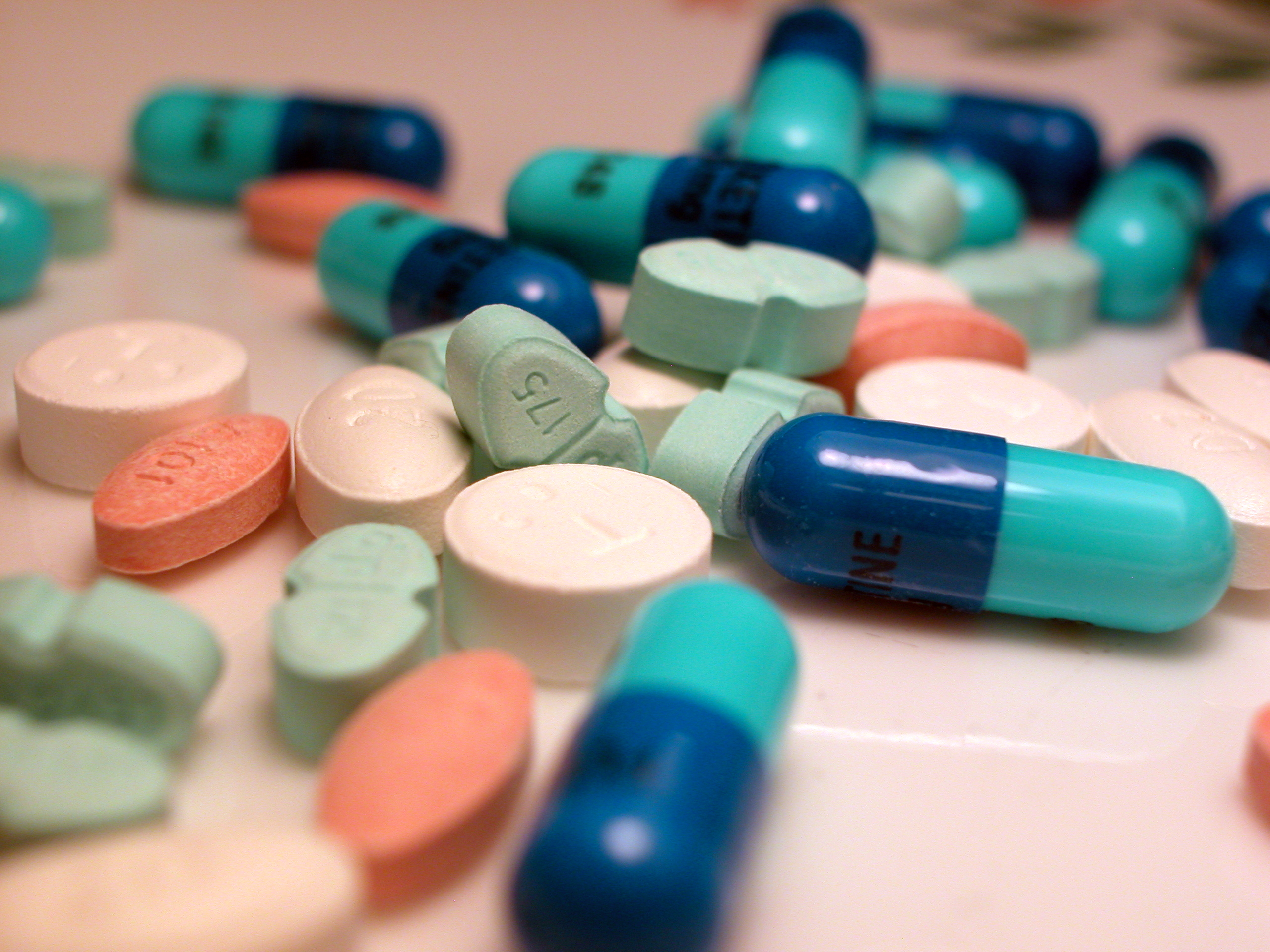
Medication
A medication (also called medicament, medicine, pharmaceutical drug, medicinal drug or simply drug) is a drug used to diagnose, cure, treat, or prevent disease. Drug therapy (pharmacotherapy) is an important part of the medical field and relies on the science of pharmacology for continual advancement and on pharmacy for appropriate management. Drugs are classified in multiple ways. One of the key divisions is by level of control, which distinguishes prescription drugs (those that a pharmacist dispenses only on the order of a physician, physician assistant, or qualified nurse) from over-the-counter drugs (those that consumers can order for themselves). Another key distinction is between traditional small molecule drugs, usually derived from chemical synthesis, and biopharmaceuticals, which include recombinant proteins, vaccines, blood products used therapeutically (such as IVIG), gene therapy, monoclonal antibodies and cell therapy (for instance, stem cell therapies) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quality Control
Quality control (QC) is a process by which entities review the quality of all factors involved in production. ISO 9000 defines quality control as "a part of quality management focused on fulfilling quality requirements". This approach places emphasis on three aspects (enshrined in standards such as ISO 9001): # Elements such as controls, job management, defined and well managed processes, performance and integrity criteria, and identification of records # Competence, such as knowledge, skills, experience, and qualifications # Soft elements, such as personnel, integrity, confidence, organizational culture, motivation, team spirit, and quality relationships. Inspection is a major component of quality control, where physical product is examined visually (or the end results of a service are analyzed). Product inspectors will be provided with lists and descriptions of unacceptable product defects such as cracks or surface blemishes for example. History and introduction Ea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microbe
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in older texts. The informal synonym ''microbe'' () comes from μικρός, mikrós, "small" and βίος, bíos, "life". is an organism of microscopic size, which may exist in its single-celled form or as a colony of cells. The possible existence of unseen microbial life was suspected from ancient times, such as in Jain scriptures from sixth century BC India. The scientific study of microorganisms began with their observation under the microscope in the 1670s by Anton van Leeuwenhoek. In the 1850s, Louis Pasteur found that microorganisms caused food spoilage, debunking the theory of spontaneous generation. In the 1880s, Robert Koch discovered that microorganisms caused the diseases tuberculosis, cholera, diphtheria, and anthrax. Because mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Title 21 Of The Code Of Federal Regulations
Title 21 is the portion of the Code of Federal Regulations that governs food and drugs within the United States for the Food and Drug Administration (FDA), the Drug Enforcement Administration (DEA), and the Office of National Drug Control Policy (ONDCP). It is divided into three chapters: * Chapter I — Food and Drug Administration * Chapter II — Drug Enforcement Administration * Chapter III — Office of National Drug Control Policy Chapter I Most of the Chapter I regulations are based on the Federal Food, Drug, and Cosmetic Act. Notable sections: * 11 — electronic records and electronic signature related * 50 Protection of human subjects in clinical trials * 54 Financial disclosure by clinical investigators * 56 Institutional review boards that oversee clinical trials * 58 Good laboratory practices (GLP) for nonclinical studies The 100 series are regulations pertaining to food: * 101, especially 101.9 — Nutrition facts label related ** (c)(2)(ii) — Requirement to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Organization For Standardization
The International Organization for Standardization (ISO ) is an international standard development organization composed of representatives from the national standards organizations of member countries. Membership requirements are given in Article 3 of the ISO Statutes. ISO was founded on 23 February 1947, and (as of November 2022) it has published over 24,500 international standards covering almost all aspects of technology and manufacturing. It has 809 Technical committees and sub committees to take care of standards development. The organization develops and publishes standardization in all technical and nontechnical fields other than electrical and electronic engineering, which is handled by the IEC.Editors of Encyclopedia Britannica. 3 June 2021.International Organization for Standardization" ''Encyclopedia Britannica''. Retrieved 2022-04-26. It is headquartered in Geneva, Switzerland, and works in 167 countries . The three official languages of the ISO are English, Fren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Most Probable Number
The most probable number method, otherwise known as the method of Poisson zeroes, is a method of getting quantitative data on concentrations of discrete items from positive/negative (incidence) data. There are many discrete entities that are easily detected but difficult to count. Any sort of amplification reaction or catalysis reaction obliterates easy quantification but allows presence to be detected very sensitively. Common examples include microorganism growth, enzyme action, or catalytic chemistry. The MPN method involves taking the original solution or sample, and subdividing it by orders of magnitude (frequently 10× or 2×), and assessing presence/absence in multiple subdivisions. The degree of dilution at which absence begins to appear indicates that the items have been diluted so much that there are many subsamples in which none appear. A suite of replicates at any given concentration allow finer resolution, to use the number of positive and negative samples to est ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colony Forming Unit
In microbiology, colony-forming unit (CFU, cfu or Cfu) is a unit which estimates the number of microbial cells (bacteria, fungi, viruses etc.) in a sample that are viable, able to multiply via binary fission under the controlled conditions. Counting with colony-forming units requires culturing the microbes and counts only viable cells, in contrast with microscopic examination which counts all cells, living or dead. The visual appearance of a colony in a cell culture requires significant growth, and when counting colonies, it is uncertain if the colony arose from one cell or a group of cells. Expressing results as colony-forming units reflects this uncertainty. Theory The purpose of plate counting is to estimate the number of cells present based on their ability to give rise to colonies under specific conditions of nutrient medium, temperature and time. Theoretically, one viable cell can give rise to a colony through replication. However, solitary cells are the exception in natur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Healthcare-associated Infections
A hospital-acquired infection, also known as a nosocomial infection (from the Greek , meaning "hospital"), is an infection that is acquired in a hospital or other health care facility. To emphasize both hospital and nonhospital settings, it is sometimes instead called a healthcare–associated infection. Such an infection can be acquired in hospital, nursing home, rehabilitation facility, outpatient clinic, diagnostic laboratory or other clinical settings. Infection is spread to the susceptible patient in the clinical setting by various means. Health care staff also spread infection, in addition to contaminated equipment, bed linens, or air droplets. The infection can originate from the outside environment, another infected patient, staff that may be infected, or in some cases, the source of the infection cannot be determined. In some cases the microorganism originates from the patient's own skin microbiota, becoming opportunistic after surgery or other procedures that compromise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hospital-acquired Infection
A hospital-acquired infection, also known as a nosocomial infection (from the Greek , meaning "hospital"), is an infection that is acquired in a hospital or other health care facility. To emphasize both hospital and nonhospital settings, it is sometimes instead called a healthcare–associated infection. Such an infection can be acquired in hospital, nursing home, rehabilitation facility, outpatient clinic, diagnostic laboratory or other clinical settings. Infection is spread to the susceptible patient in the clinical setting by various means. Health care staff also spread infection, in addition to contaminated equipment, bed linens, or air droplets. The infection can originate from the outside environment, another infected patient, staff that may be infected, or in some cases, the source of the infection cannot be determined. In some cases the microorganism originates from the patient's own skin microbiota, becoming opportunistic after surgery or other procedures that compromise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HEPA
HEPA (, high-efficiency particulate air) filter, also known as high-efficiency particulate absorbing filter and high-efficiency particulate arrestance filter, is an efficiency standard of air filters. Filters meeting the HEPA standard must satisfy certain levels of efficiency. Common standards require that a HEPA air filter must remove—from the air that passes through—at least 99.95% (ISO, European Standard) or 99.97% (ASME, U.S. DOE) of particles whose diameter is equal to 0.3 μm, with the filtration efficiency increasing for particle diameters both less than and greater than 0.3 μm. HEPA filters capture pollen, dirt, dust, moisture, bacteria (0.2-2.0 μm), virus (0.02-0.3 μm), and submicron liquid aerosol (0.02-0.5 μm). Some microorganisms, for example, ''Aspergillus niger'', ''Penicillium citrinum'', ''Staphylococcus epidermidis'', and ''Bacillus subtilis'' are captured by HEPA filters with photocatalytic oxidation (PCO). A HEPA filter is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |