|
Bromodichloromethane
Bromodichloromethane is a trihalomethane with formula C H Br Cl2. Bromodichloromethane has formerly been used as a flame retardant, and a solvent for fats and waxes and because of its high density for mineral separation. Now it is only used as a reagent or intermediate in organic chemistry. Bromodichloromethane can also occur in municipally-treated drinking water Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, a ... as a by-product of the chlorine disinfection process.Agency for Toxic Substances & Disease Registry, Accessed 07/10/2012, http://www.atsdr.cdc.gov/toxfaqs/tf.asp?id=707&tid=127 Notes External links * Bromodichloromethane at The Carcinogenic Potency DatabaseToxicological Profile at ATSDR Organochlorides Halomethanes IARC Group 2B carcinogens Organobromides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trihalomethane
In chemistry, trihalomethanes (THMs) are chemical compounds in which three of the four hydrogen atoms of methane () are replaced by halogen atoms. Many trihalomethanes find uses in industry as solvents or refrigerants. THMs are also environmental pollutants, and many are considered carcinogenic. Trihalomethanes with all the same halogen atoms are called haloforms. Table of common trihalomethanes Industrial uses Only chloroform has significant applications of the haloforms. In the predominant application, chloroform is required for the production of tetrafluoroethylene, precursor to teflon. Chloroform is fluorinated by reaction with hydrogen fluoride to produce chlorodifluoromethane (R-22). Pyrolysis of chlorodifluoromethane (at 550-750 °C) yields TFE, with difluorocarbene as an intermediate. :CHCl3 + 2 HF -> CHClF2 + 2 HCl :2 CHClF2 -> C2F4 + 2 HCl Refrigerants and solvents Trifluoromethane and chlorodifluoromethane are both used as refrigerants. Trihalomethanes re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halomethanes
Halomethane compounds are derivatives of methane () with one or more of the hydrogen atoms replaced with halogen atoms ( F, Cl, Br, or I). Halomethanes are both naturally occurring, especially in marine environments, and human-made, most notably as refrigerants, solvents, propellants, and fumigants. Many, including the chlorofluorocarbons, have attracted wide attention because they become active when exposed to ultraviolet light found at high altitudes and destroy the Earth's protective ozone layer. Structure and properties Like methane itself, halomethanes are tetrahedral molecules. The halogen atoms differ greatly in size and charge from hydrogen and from each other. Consequently, most halomethanes deviate from the perfect tetrahedral symmetry of methane.Günter Siegemund, Werner Schwertfeger, Andrew Feiring, Bruce Smart, Fred Behr, Herward Vogel, Blaine McKusick “Fluorine Compounds, Organic” Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. The ph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organochlorides
An organochloride, organochlorine compound, chlorocarbon, or chlorinated hydrocarbon is an organic compound containing at least one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) provides common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious. Physical and chemical properties Chlorination modifies the physical properties of hydrocarbons in several ways. These compounds are typically denser than water due to the higher atomic weight of chlorine versus hydrogen. Aliphatic organochlorides are often alkylating agents as chlorine can act as a leaving group, which can result in cellular damage. Natural occurrence Many organochlorine compounds have been isolate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water Chlorination
Water chlorination is the process of adding chlorine or chlorine compounds such as sodium hypochlorite to water. This method is used to kill bacteria, viruses and other microbes in water. In particular, chlorination is used to prevent the spread of waterborne diseases such as cholera, dysentery, and typhoid. History In a paper published in 1894, it was formally proposed to add chlorine to water to render it "germ-free". Two other authorities endorsed this proposal and published it in many other papers in 1895. Early attempts at implementing water chlorination at a water treatment plant were made in 1893 in Hamburg, Germany. In 1897 the town of Maidstone, England was the first to have its entire water supply treated with chlorine. Permanent water chlorination began in 1905, when a faulty slow sand filter and a contaminated water supply caused a serious typhoid fever epidemic in Lincoln, England. Alexander Cruickshank Houston used chlorination of the water to stop the epidemic. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drinking Water
Drinking water is water that is used in drink or food preparation; potable water is water that is safe to be used as drinking water. The amount of drinking water required to maintain good health varies, and depends on physical activity level, age, health-related issues, and environmental conditions. This 2004 article focuses on the USA context and uses data collected from the US military. Recent work showed that the most important driver of water turnover which is closely linked to water requirements is energy expenditure. For those who work in a hot climate, up to a day may be required. Typically in developed countries, tap water meets drinking water quality standards, even though only a small proportion is actually consumed or used in food preparation. Other typical uses for tap water include washing, toilets, and irrigation. Greywater may also be used for toilets or irrigation. Its use for irrigation however may be associated with risks. Water may also be unacceptable due to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes physical and chemical properties, and evaluation of chemical reactivity to understand their behavior. The study of organic reactions includes the chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical ( in silico) study. The range of chemicals studied in organic chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (included in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Intermediate
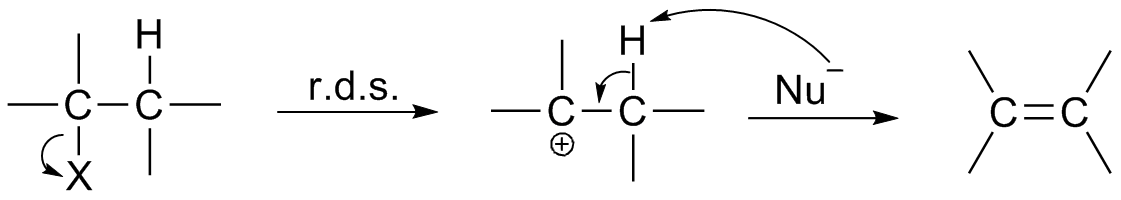
In chemistry, a reaction intermediate or an intermediate is a molecular entity that is formed from the reactants (or preceding intermediates) but is consumed in further reactions in stepwise chemical reactions that contain multiple elementary steps. Intermediates are the reaction product of one elementary step, but do not appear in the chemical equation for an overall chemical equation. For example, consider this hypothetical stepwise reaction: :A + B -> C + D The reaction includes two elementary steps: :A + B -> X :X -> C + D In this example, X is a reaction intermediate. IUPAC definition The IUPAC Gold Book defines an ''intermediate'' as a compound that has a lifetime greater than a molecular vibration that is formed (directly or indirectly) from the reactants and reacts further to give (either directly or indirectly) the products of a chemical reaction. The lifetime condition distinguishes true, chemically distinct intermediates from vibrational states or such transition st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a substance ''consumed'' in the course of a chemical reaction. ''Solvents'', though involved in the reaction mechanism, are usually not called reactants. Similarly, ''catalysts'' are not consumed by the reaction, so they are not reactants. In biochemistry, especially in connection with enzyme-catalyzed reactions, the reactants are commonly called substrates. Definitions Organic chemistry In organic chemistry, the term "reagent" denotes a chemical ingredient (a compound or mixture, typically of inorganic or small organic molecules) introduced to cause the desired transformation of an organic substance. Examples include the Collins reagent, Fenton's reagent, and Grignard reagents. Analytical chemistry In analytical chemistry, a reagent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Waxes
Waxes are a diverse class of organic compounds that are lipophilic, malleable solids near ambient temperatures. They include higher alkanes and lipids, typically with melting points above about 40 °C (104 °F), melting to give low viscosity liquids. Waxes are insoluble in water but soluble in nonpolar organic solvents such as hexane, benzene and chloroform. Natural waxes of different types are produced by plants and animals and occur in petroleum. Chemistry Waxes are organic compounds that characteristically consist of long aliphatic alkyl chains, although aromatic compounds may also be present. Natural waxes may contain unsaturated bonds and include various functional groups such as fatty acids, primary and secondary alcohols, ketones, aldehydes and fatty acid esters. Synthetic waxes often consist of homologous series of long-chain aliphatic hydrocarbons (alkanes or paraffins) that lack functional groups. Plant and animal waxes Waxes are synthesized by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and ''plus'' (+) and ''minus'' (−) signs. These are limited to a single typographic line of symbols, which may include Subscript and superscript, subscripts and superscripts. A chemical formula is not a chemical nomenclature, chemical name, and it contains no words. Although a chemical formula may imply certain simple chemical structures, it is not the same as a full chemical structural formula. Chemical formulae can fully specify the structure of only the simplest of molecules and chemical substances, and are generally more limited in power than chemical names and structural formulae. The simplest types of chemical formulae are called ''empirical formulae'', which use letters and numbers ind ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FAT (gene)
Protocadherin FAT1 is a protein that in humans is encoded by the ''FAT1'' gene. Function This gene is an ortholog of the ''Drosophila'' fat gene, which encodes a tumor suppressor essential for controlling cell proliferation during Drosophila development. The gene product is a member of the cadherin superfamily, a group of integral membrane proteins characterized by the presence of cadherin-type repeats. This gene is expressed at high levels in a number of fetal epithelia. Transcript variants derived from alternative splicing and/or alternative promoter usage exist, but they have not been fully described. The murine Fat1 knockout mouse is not embryonically lethal but pups die within 48-hours due to the abnormal fusion of foot processes of the podocytes within the kidney. These Fat1 knockout mice also showed partially penetrant but often severe midline defects including holoprosencephaly, microphthalmia-anophthalmia and in rare cases cyclopia. It has been shown that the EVH mot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |