|
Borenium Ion
In chemistry, a boranylium ion is an inorganic cation with the chemical formula , where R represents a non-specific substituent. Being electron-deficient, boranylium ions form adducts with Lewis bases. Boranylium ions have historical names that depend on the number of coordinated ligands: *: borinium *: borenium *: boronium Borenium ions A borenium ion is an inorganic cation with the chemical formula . In this class of molecules, the electron-deficient boron center has two valence electrons involved in sigma bonding with two ligands, while the third ligand is a two-electron donor such that the overall charge of the complex is +1. Depending on the nature of the ligands around the central boron, this positive charge can be localized on the boron center or delocalized across the entire molecule. Borenium ions can be made in a number of different ways and are of interest for applications in organic synthesis and catalysis. Synthesis Synthetic methods for preparing borenium ions incl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
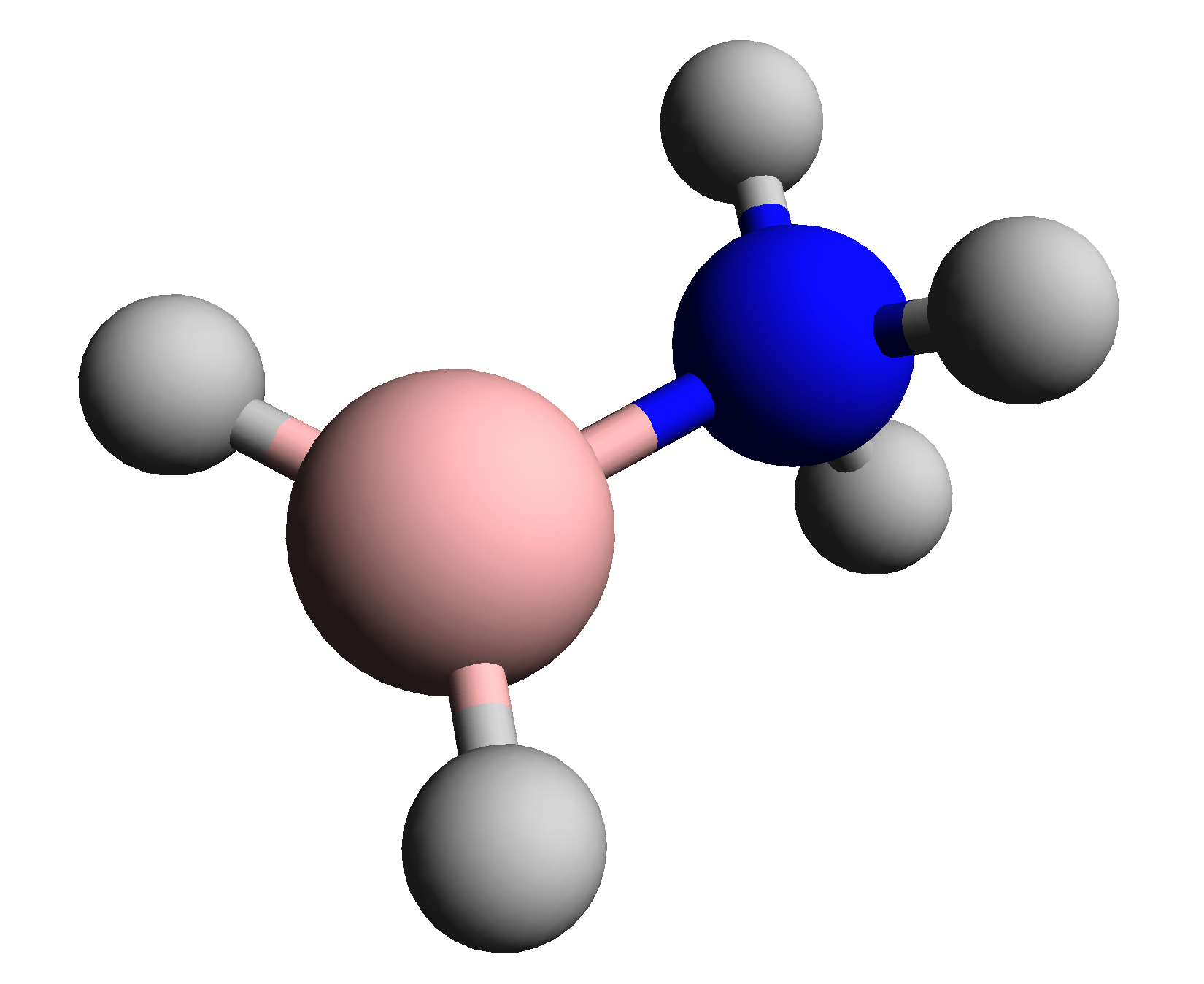
Bh2nh3
BH, Bh or bh may refer to: Medicine * Bernard-Horner syndrome, a combination of symptoms that arises when a group of nerves known as the sympathetic trunk is damaged * Borderline hypertensive, an American medical classification for cases where a person's blood pressure is elevated above normal, but not to the level considered hypertension * Bronchial hyperresponsiveness, a state characterised by easily triggered bronchospasm * Bundle of His, collection of heart muscle cells specialized for electrical conduction Science and technology * BH register, in computer architectures * Bohrium, symbol Bh, a chemical element * Boron monohydride, chemical formula BH, a chemical compound * Black hole Places * BH postcode area, a region in southern England served by Bournemouth postal sorting office * Bahrain (ISO 3166-1 country code BH) ** .bh, the Internet country code top-level domain for Bahrain * Belize's WMO and obsolete NATO country code digram * Belo Horizonte, the capital of Mina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protic Attack Mech
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labile is called a protic solvent. The molecules of such solvents readily donate protons () to solutes, often via hydrogen bonding. Water is the most common protic solvent. Conversely, polar aprotic solvents cannot donate protons but still have the ability to dissolve many salts. Methods for purification of common solvents are available See also * Autoprotolysis In chemistry, autoprotolysis is a chemical reaction in which a proton is transferred between two identical molecules, one of which acts as a Brønsted acid, releasing a proton which is accepted by the other molecule acting as a Brønsted base. ... References {{Chemical solutions Solvents ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frustrated Lewis Pair
A frustrated Lewis pair (FLP) is a compound or mixture containing a Lewis acid and a Lewis base that, because of steric hindrance, cannot combine to form a classical adduct. Many kinds of FLPs have been devised, and many simple substrates exhibit activation. The discovery that some FLPs split H2 triggered a rapid growth of research into FLPs. Because of their "unquenched" reactivity, such systems are reactive toward substrates that can undergo heterolysis. For example, many FLPs split hydrogen molecules. Thus, a mixture of tricyclohexylphosphine (PCy3) and tris(pentafluorophenyl)borane reacts with hydrogen to give the respective phosphonium and borate ions: :PCy3 + B(C6F5)3 + H2 -> PCy3 B(C6F5)3 This reactivity has been exploited to produce FLPs which catalyse hydrogenation reactions. Small molecule activation Frustrated Lewis pairs have been shown to activate many small molecules, either by inducing heterolysis or by coordination. Hydrogen The discovery that some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a Catalysis, catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gutmann–Beckett Method
In chemistry, the Gutmann–Beckett method is an experimental procedure used by chemists to assess the Lewis acidity of molecular species. Triethylphosphine oxide (, TEPO) is used as a probe molecule and systems are evaluated by 31P-NMR spectroscopy. In 1975, used 31P-NMR spectroscopy to parameterize Lewis acidity of solvents by acceptor numbers (AN).U. Mayer, V. Gutmann, and W. Gerger, "The acceptor number – a quantitative empirical parameter for the electrophilic properties of solvents", ''Monatshefte fur Chemie,'' 1975, 106, 1235–1257. doi: 10.1007/BF00913599 In 1996, Michael A. Beckett recognised its more generally utility and adapted the procedure so that it could be easily applied to molecular species, when dissolved in weakly Lewis acidic solvents.M.A. Beckett, G.C. Strickland, J.R. Holland, and K.S. Varma, "A convenient NMR method for the measurement of Lewis acidity at boron centres: correlation of reaction rates of Lewis acid initiated epoxide polymerizations wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
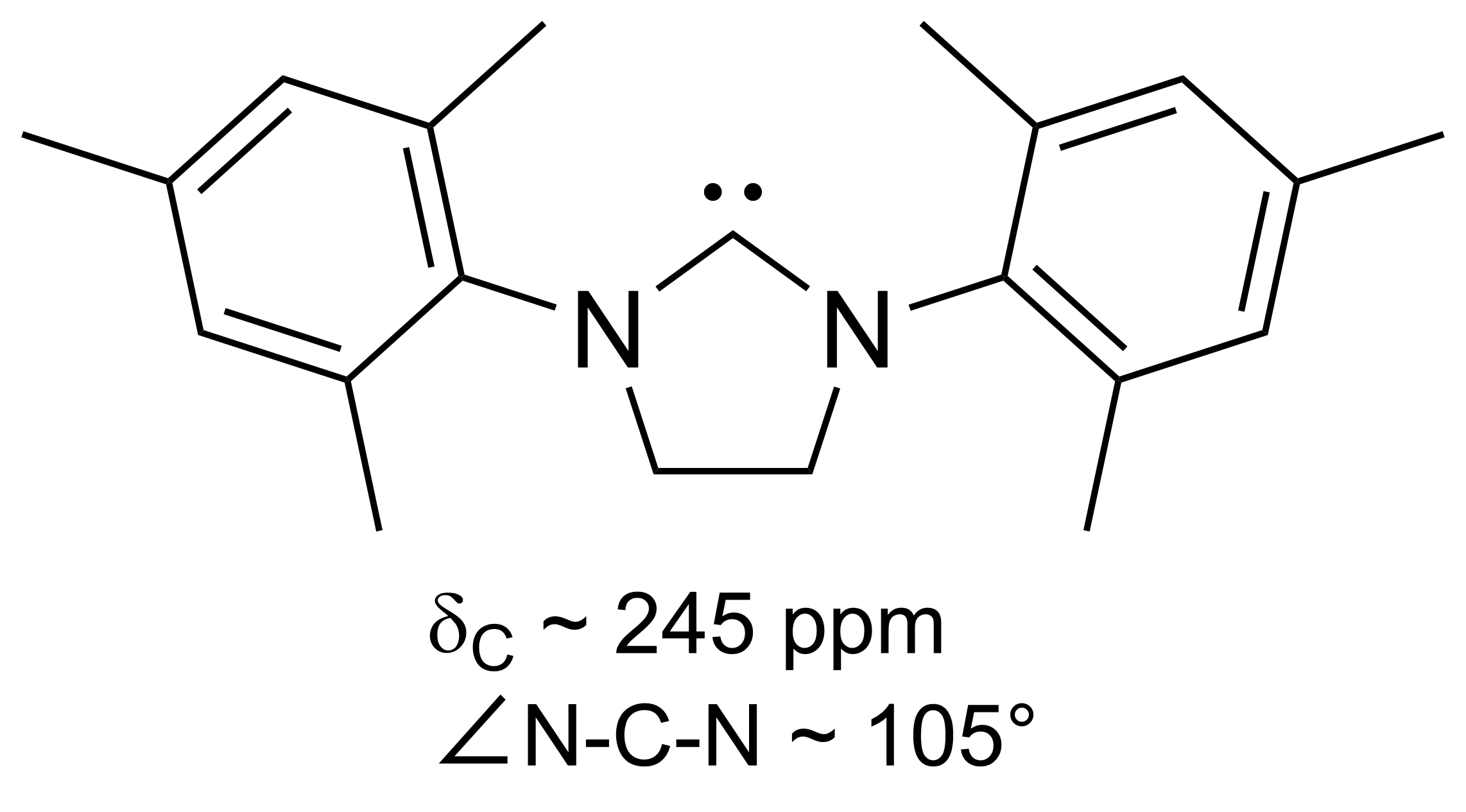
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bh2nhc Nocv
BH, Bh or bh may refer to: Medicine * Bernard-Horner syndrome, a combination of symptoms that arises when a group of nerves known as the sympathetic trunk is damaged * Borderline hypertensive, an American medical classification for cases where a person's blood pressure is elevated above normal, but not to the level considered hypertension * Bronchial hyperresponsiveness, a state characterised by easily triggered bronchospasm * Bundle of His, collection of heart muscle cells specialized for electrical conduction Science and technology * BH register, in computer architectures * Bohrium, symbol Bh, a chemical element * Boron monohydride, chemical formula BH, a chemical compound * Black hole Places * BH postcode area, a region in southern England served by Bournemouth postal sorting office * Bahrain (ISO 3166-1 country code BH) ** .bh, the Internet country code top-level domain for Bahrain * Belize's WMO and obsolete NATO country code digram * Belo Horizonte, the capital of Mina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aromaticity
In chemistry, aromaticity is a chemical property of cyclic ( ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to saturated compounds having single bonds, and other geometric or connective non-cyclic arrangements with the same set of atoms. Aromatic rings are very stable and do not break apart easily. Organic compounds that are not aromatic are classified as aliphatic compounds—they might be cyclic, but only aromatic rings have enhanced stability. The term ''aromaticity'' with this meaning is historically related to the concept of having an aroma, but is a distinct property from that meaning. Since the most common aromatic compounds are derivatives of benzene (an aromatic hydrocarbon common in petroleum and its distillates), the word ''aromatic'' occasionally refers informally to benzene derivatives, and so it was first defined. Nevertheless, many non-be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density Functional Theory
Density-functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body systems, in particular atoms, molecules, and the condensed phases. Using this theory, the properties of a many-electron system can be determined by using functionals, i.e. functions of another function. In the case of DFT, these are functionals of the spatially dependent electron density. DFT is among the most popular and versatile methods available in condensed-matter physics, computational physics, and computational chemistry. DFT has been very popular for calculations in solid-state physics since the 1970s. However, DFT was not considered accurate enough for calculations in quantum chemistry until the 1990s, when the approximations used in the theory were greatly refined to better model the exchange and correlation interactions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordinate Covalent Bond
In coordination chemistry, a coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the two electrons derive from the same atom. The bonding of metal ions to ligands involves this kind of interaction. This type of interaction is central to Lewis acid–base theory. Coordinate bonds are commonly found in coordination compounds. Examples Coordinate covalent bonding is ubiquitous. In all metal aquo-complexes (H2O)''n'''m''+, the bonding between water and the metal cation is described as a coordinate covalent bond. Metal-ligand interactions in most organometallic compounds and most coordination compounds are described similarly. The term ''dipolar bond'' is used in organic chemistry for compounds such as amine oxides for which the electronic structure can be described in terms of the basic amine donating two electrons to an oxygen atom. : → O The arrow → indicates that both ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.png)

