|
Bentonitic
Bentonite () is an absorbent swelling clay consisting mostly of montmorillonite (a type of smectite) which can either be Na-montmorillonite or Ca-montmorillonite. Na-montmorillonite has a considerably greater swelling capacity than Ca-montmorillonite. Bentonite usually forms from the weathering of volcanic ash in seawater, or by hydrothermal circulation through the porosity of volcanic ash beds, which converts (devitrification) the volcanic glass ( obsidian, rhyolite, dacite) present in the ash into clay minerals. In the mineral alteration process, a large fraction (up to 40-50 wt.%) of amorphous silica is dissolved and leached away, leaving the bentonite deposit in place. Bentonite beds are white or pale blue or green (traces of reduced ) in fresh exposures, turning to a cream color and then yellow, red, or brown (traces of oxidized ) as the exposure is weathered further. As a swelling clay, bentonite has the ability to absorb large quantities of water, which increa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benstonite
Benstonite is a mineral with formula Ba6Ca6Mg(CO3)13. Discovered in 1954, the mineral was described in 1961 and named after Orlando J. Benston (1901–1966). Description and occurrence Benstonite is translucent and white, pale yellow, or pale yellow-brown in color. The mineral occurs as cleavable masses; cleavage fragments are nearly perfectly rhombohedral in shape. Cleavage faces are up to across and slightly curved. On large specimens, the faces exhibit a mosaic structure similar to that in some specimens of dolomite and siderite. Benstonite fluoresces red or yellow under x-rays and longwave and shortwave ultraviolet. The mineral also exhibits strong red phosphorescence. Benstonite is known to occur in Canada, China, India, Italy, Namibia, Russia, Sweden, and the United States. It occurs in association with alstonite, barite, barytocalcite, calcite, daqingshanite, fluorite, huntite, monazite, phlogopite, pyrite, sphalerite, strontianite, and quartz. Synthesis The mineral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dacite
Dacite () is a volcanic rock formed by rapid solidification of lava that is high in silica and low in alkali metal oxides. It has a fine-grained (aphanitic) to porphyritic texture and is intermediate in composition between andesite and rhyolite. It is composed predominantly of plagioclase feldspar and quartz. Dacite is relatively common, occurring in many tectonic settings. It is associated with andesite and rhyolite as part of the subalkaline volcanic rock, subalkaline tholeiite, tholeiitic and calc-alkaline magma series. Composition Dacite consists mostly of plagioclase feldspar and quartz with biotite, hornblende, and pyroxene (augite or enstatite). The quartz appears as rounded, corroded phenocrysts, or as an element of the ground-mass. The plagioclase in dacite ranges from oligoclase to andesine and labradorite. Sanidine occurs, although in small proportions, in some dacites, and when abundant gives rise to rocks that form rhyodacite, transitions to the rhyolites. The rel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kaolinite
Kaolinite ( ) is a clay mineral, with the chemical composition Al2 Si2 O5( OH)4. It is an important industrial mineral. It is a layered silicate mineral, with one tetrahedral sheet of silica () linked through oxygen atoms to one octahedral sheet of alumina () octahedra. Rocks that are rich in kaolinite are known as kaolin () or china clay. Kaolin is occasionally referred to by the antiquated term lithomarge, from the Ancient Greek ''litho-'' and Latin ''marga'', meaning 'stone of marl'. Presently the name lithomarge can refer to a compacted, massive form of kaolin. The name ''kaolin'' is derived from Gaoling (), a Chinese village near Jingdezhen in southeastern China's Jiangxi Province. The name entered English in 1727 from the French version of the word: , following François Xavier d'Entrecolles's reports on the making of Jingdezhen porcelain. Kaolinite has a low shrink–swell capacity and a low cation-exchange capacity (1–15 meq/100 g). It is a soft, earthy, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binder (material)
A binder or binding agent is any material or substance that holds or draws other materials together to form a cohesive whole mechanically, chemically, by adhesion or cohesion. In a more narrow sense, binders are liquid or dough-like substances that harden by a chemical or physical process and bind fibres, filler powder and other particles added into it. Examples include glue, adhesive and thickening. Examples of mechanical binders are bond stones in masonry and tie beams in timber framing. Classification Binders are loosely classified as organic ( bitums, animal and plant glues, polymers) and inorganic ( lime, cement, gypsum, liquid glass, etc.). These can be either metallic or ceramic as well as polymeric depending on the nature of the main material. For example, in the compound WC-Co (Tungsten Carbide used in cutting tools) Co constitutes the binding agent for the WC particles. Based on their chemical resistance, binders are classified by the field of use: non-hydra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adsorbent
Adsorption is the adhesion of atoms, ions or molecules from a gas, liquid or dissolved solid to a surface. This process creates a film of the ''adsorbate'' on the surface of the ''adsorbent''. This process differs from absorption, in which a fluid (the ''absorbate'') is dissolved by or permeates a liquid or solid (the ''absorbent''). Adsorption is a '' surface phenomenon'', while absorption involves the whole volume of the material, although adsorption does often precede absorption. The term ''sorption'' encompasses both processes, while ''desorption'' is the reverse of it. Like surface tension, adsorption is a consequence of surface energy. In a bulk material, all the bonding requirements (be they ionic, covalent or metallic) of the constituent atoms of the material are fulfilled by other atoms in the material. However, atoms on the surface of the adsorbent are not wholly surrounded by other adsorbent atoms and therefore can attract adsorbates. The exact nature of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mineral
In geology and mineralogy, a mineral or mineral species is, broadly speaking, a solid chemical compound with a fairly well-defined chemical composition and a specific crystal structure that occurs naturally in pure form.John P. Rafferty, ed. (2011): Minerals'; p. 1. In the series ''Geology: Landforms, Minerals, and Rocks''. Rosen Publishing Group. The geological definition of mineral normally excludes compounds that occur only in living organisms. However, some minerals are often biogenic (such as calcite) or are organic compounds in the sense of chemistry (such as mellite). Moreover, living organisms often synthesize inorganic minerals (such as hydroxylapatite) that also occur in rocks. The concept of mineral is distinct from rock, which is any bulk solid geologic material that is relatively homogeneous at a large enough scale. A rock may consist of one type of mineral, or may be an aggregate of two or more different types of minerals, spacially segregated into distinct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicate Minerals
Silicate minerals are rock-forming minerals made up of silicate groups. They are the largest and most important class of minerals and make up approximately 90 percent of Earth's crust. In mineralogy, silica (silicon dioxide, ) is usually considered a silicate mineral. Silica is found in nature as the mineral quartz, and its polymorphs. On Earth, a wide variety of silicate minerals occur in an even wider range of combinations as a result of the processes that have been forming and re-working the crust for billions of years. These processes include partial melting, crystallization, fractionation, metamorphism, weathering, and diagenesis. Living organisms also contribute to this geologic cycle. For example, a type of plankton known as diatoms construct their exoskeletons ("frustules") from silica extracted from seawater. The frustules of dead diatoms are a major constituent of deep ocean sediment, and of diatomaceous earth. General structure A silicate mineral is general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It has a great affinity towards oxygen, and forms a protective layer of oxide on the surface when exposed to air. Aluminium visually resembles silver, both in its color and in its great ability to reflect light. It is soft, non-magnetic and ductile. It has one stable isotope, 27Al; this isotope is very common, making aluminium the twelfth most common element in the Universe. The radioactivity of 26Al is used in radiodating. Chemically, aluminium is a post-transition metal in the boron group; as is common for the group, aluminium forms compounds primarily in the +3 oxidation state. The aluminium cation Al3+ is small and highly charged; as such, it is polarizing, and bonds aluminium forms tend towards covalency. The strong affinity tow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drilling Mud
In geotechnical engineering, drilling fluid, also called drilling mud, is used to aid the drilling of boreholes into the earth. Often used while drilling oil and natural gas wells and on exploration drilling rigs, drilling fluids are also used for much simpler boreholes, such as water wells. One of the functions of drilling mud is to carry cuttings out of the hole. The three main categories of drilling fluids are: water-based muds (WBs), which can be dispersed and non-dispersed; non-aqueous muds, usually called oil-based muds (OBs); and gaseous drilling fluid, in which a wide range of gases can be used. Along with their formatives, these are used along with appropriate polymer and clay additives for drilling various oil and gas formations. The main functions of drilling fluids include providing hydrostatic pressure to prevent formation fluids from entering into the well bore, keeping the drill bit cool and clean during drilling, carrying out drill cuttings, and suspending t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogenation, C=C (and other) bonds ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redox
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate (chemistry), substrate change. Oxidation is the loss of Electron, electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. There are two classes of redox reactions: * ''Electron-transfer'' – Only one (usually) electron flows from the reducing agent to the oxidant. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * ''Atom transfer'' – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidation reactions are commonly associated with the formation of oxides, other chemical species can serve the same function. In hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
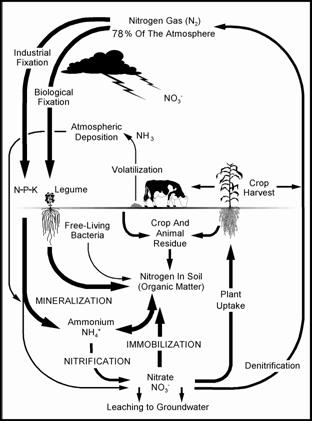
Leaching (agriculture)
In agriculture, leaching is the loss of water-soluble plant nutrients from the soil, due to rain and irrigation. Soil structure, crop planting, type and application rates of fertilizers, and other factors are taken into account to avoid excessive nutrient loss. Leaching may also refer to the practice of applying a small amount of excess irrigation where the water has a high salt content to avoid salts from building up in the soil (salinity control). Where this is practiced, drainage must also usually be employed, to carry away the excess water. Leaching is a natural environment concern when it contributes to groundwater contamination. As water from rain, flooding, or other sources seeps into the ground, it can dissolve chemicals and carry them into the underground water supply. Of particular concern are hazardous waste dumps and landfills, and, in agriculture, excess fertilizer, improperly stored animal manure, and biocides (e.g. pesticides, fungicides, insecticides and herb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |