|
Bathochromic Shift
Bathochromic shift (from Greek βαθύς ''bathys'', "deep"; and χρῶμα ''chrōma'', "color"; hence less common alternate spelling "bathychromic") is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength (lower frequency). Because the red color in the visible spectrum has a longer wavelength than most other colors, the effect is also commonly called a '' red shift''. Hypsochromic shift is a change to shorter wavelength (higher frequency). Conditions It can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism. A series of structurally-related molecules in a substitution series can also show a bathochromic shift. Bathochromic shift is a phenomenon seen in ''molecular'' spectra, not ''atomic'' spectra; it is thus more common to speak of the movement of the peaks in the spectrum rather than lines. :\Delta\lambda = \la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Absorption Spectrum
Absorption spectroscopy refers to spectroscopic techniques that measure the absorption of radiation, as a function of frequency or wavelength, due to its interaction with a sample. The sample absorbs energy, i.e., photons, from the radiating field. The intensity of the absorption varies as a function of frequency, and this variation is the absorption spectrum. Absorption spectroscopy is performed across the electromagnetic spectrum. Absorption spectroscopy is employed as an analytical chemistry tool to determine the presence of a particular substance in a sample and, in many cases, to quantify the amount of the substance present. Infrared and ultraviolet–visible spectroscopy are particularly common in analytical applications. Absorption spectroscopy is also employed in studies of molecular and atomic physics, astronomical spectroscopy and remote sensing. There is a wide range of experimental approaches for measuring absorption spectra. The most common arrangement is to dire ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvatochromism
In chemistry, solvatochromism is the phenomenon observed when the colour due to a solute is different when that solute is dissolved in different solvents. The solvatochromic effect is the way the spectrum of a substance (the solute) varies when the substance is dissolved in a variety of solvents. In this context, the dielectric constant and hydrogen bonding capacity are the most important properties of the solvent. With various solvents there is a different effect on the electronic ground state and excited state of the solute, so that the size of energy gap between them changes as the solvent changes. This is reflected in the absorption or emission spectrum of the solute as differences in the position, intensity, and shape of the spectroscopic bands. When the spectroscopic band occurs in the visible part of the spectrum solvatochromism is observed as a change of colour. This is illustrated by Reichardt's dye, as shown in the image. Negative solvatochromism corresponds to a hypsoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvatochromism
In chemistry, solvatochromism is the phenomenon observed when the colour due to a solute is different when that solute is dissolved in different solvents. The solvatochromic effect is the way the spectrum of a substance (the solute) varies when the substance is dissolved in a variety of solvents. In this context, the dielectric constant and hydrogen bonding capacity are the most important properties of the solvent. With various solvents there is a different effect on the electronic ground state and excited state of the solute, so that the size of energy gap between them changes as the solvent changes. This is reflected in the absorption or emission spectrum of the solute as differences in the position, intensity, and shape of the spectroscopic bands. When the spectroscopic band occurs in the visible part of the spectrum solvatochromism is observed as a change of colour. This is illustrated by Reichardt's dye, as shown in the image. Negative solvatochromism corresponds to a hypsoc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromism
In chemistry, chromism is a process that induces a change, often reversible, in the colors of compounds. In most cases, chromism is based on a change in the electron states of molecules, especially the π- or d-electron state, so this phenomenon is induced by various external stimuli which can alter the electron density of substances. It is known that there are many natural compounds that have chromism, and many artificial compounds with specific chromism have been synthesized to date. It is usually synonymous with chromotropism, the (reversible) change in color of a substance due to the physical and chemical properties of its ambient surrounding medium, such as temperature and pressure, light, solvent, and presence of ions and electrons. Chromism is classified by what kind of stimuli are used. Examples of the major kinds of chromism are as follows. * thermochromism is chromism that is induced by heat, that is, a change of temperature. This is the most common chromism of all. * pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spectroradiometer
A spectroradiometer is a light measurement tool that is able to measure both the wavelength and amplitude of the light emitted from a light source. Spectrometers discriminate the wavelength based on the position the light hits at the detector array allowing the full spectrum to be obtained with a single acquisition. Most spectrometers have a base measurement of counts which is the un-calibrated reading and is thus impacted by the sensitivity of the detector to each wavelength. By applying a calibration, the spectrometer is then able to provide measurements of spectral irradiance, spectral radiance and/or spectral flux. This data is also then used with built in or PC software and numerous algorithms to provide readings or Irradiance (W/cm2), Illuminance (lux or fc), Radiance (W/sr), Luminance (cd), Flux (Lumens or Watts), Chromaticity, Color Temperature, Peak and Dominant Wavelength. Some more complex spectrometer software packages also allow calculation of PAR μmol/m2/s, Metamerism, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tristimulus Colorimeter
A tristimulus colorimeter, colloquially shortened to ''colorimeter'', is used in digital imaging to profile and calibrate output devices. It takes a limited number of wideband spectral energy readings along the visible spectrum by using filtered photodetectors; e.g. silicon photodiodes. A colorimeter with the known value of absolute error allows measuring (x,y)-chromaticity coordinates in red, green, blue and white colors. Measured values are used for calculation of LCD profile coefficients. Originally, three glass filters whose transmittance spectra mimicked the CIE color matching functions (shown below) were employed. A filter bank may be used to decompose the individual color matching functions if more accuracy is desired. A camera or colorimeter is said to be ''colorimetric'' if it satisfies the by (1868–1945) (also called the " Maxwell–Ives criterion"), reducing observer metamerism color errors, if the product of the spectral responsivity of the photoreceptor and th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substitution (chemistry)
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions are of prime importance in organic chemistry. Substitution reactions in organic chemistry are classified either as electrophilic or nucleophilic depending upon the reagent involved, whether a reactive intermediate involved in the reaction is a carbocation, a carbanion or a free radical, and whether the substrate is aliphatic or aromatic. Detailed understanding of a reaction type helps to predict the product outcome in a reaction. It also is helpful for optimizing a reaction with regard to variables such as temperature and choice of solvent. A good example of a substitution reaction is halogenation. When chlorine gas (Cl2) is irradiated, some of the molecules are split into two chlorine radicals (Cl•), whose free electrons are strongly nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Polarity
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end. Polar molecules must contain one or more polar bonds due to a difference in electronegativity between the bonded atoms. Molecules containing polar bonds have no molecular polarity if the bond dipoles cancel each other out by symmetry. Polar molecules interact through dipole–dipole intermolecular forces and hydrogen bonds. Polarity underlies a number of physical properties including surface tension, solubility, and melting and boiling points. Polarity of bonds Not all atoms attract electrons with the same force. The amount of "pull" an atom exerts on its electrons is called its electronegativity. Atoms with high electronegativitiessuch as fluorine, oxygen, and nitrogenexert a greater pull on electrons than atoms with lower electronegativities such as alkali metals and alkaline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
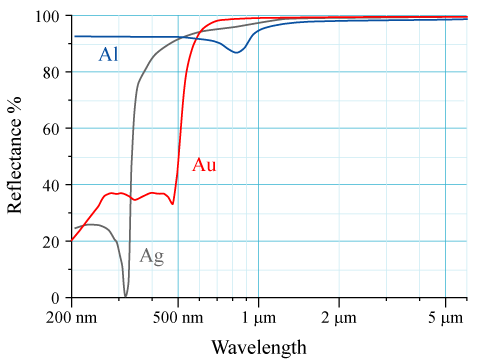
Reflectance
The reflectance of the surface of a material is its effectiveness in reflecting radiant energy. It is the fraction of incident electromagnetic power that is reflected at the boundary. Reflectance is a component of the response of the electronic structure of the material to the electromagnetic field of light, and is in general a function of the frequency, or wavelength, of the light, its polarization, and the angle of incidence. The dependence of reflectance on the wavelength is called a ''reflectance spectrum'' or ''spectral reflectance curve''. Mathematical definitions Hemispherical reflectance The ''hemispherical reflectance'' of a surface, denoted , is defined as R = \frac, where is the radiant flux ''reflected'' by that surface and is the radiant flux ''received'' by that surface. Spectral hemispherical reflectance The ''spectral hemispherical reflectance in frequency'' and ''spectral hemispherical reflectance in wavelength'' of a surface, denoted and respectively, are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypsochromic Shift
Hypsochromic shift (from ancient Greek ὕψος (upsos) "height"; and χρῶμα ''chrōma'', "color") is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a shorter wavelength (higher frequency). Because the blue color in the visible spectrum has a shorter wavelength than most other colors, this effect is also commonly called a blue shift. This can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism. A series of structurally related molecules in a substitution series can also show a hypsochromic shift. Hypsochromic shift is a phenomenon seen in ''molecular'' spectra, not ''atomic'' spectra - it is thus more common to speak of the movement of the peaks in the spectrum rather than lines. :\Delta\lambda = \lambda^_ - \lambda^_ where \lambda is the wavelength of the spectral peak of interest and \lambda^_ > \lambda^_ For example, β ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Redshift
In physics, a redshift is an increase in the wavelength, and corresponding decrease in the frequency and photon energy, of electromagnetic radiation (such as light). The opposite change, a decrease in wavelength and simultaneous increase in frequency and energy, is known as a negative redshift, or blueshift. The terms derive from the colours red and blue which form the extremes of the visible light spectrum. In astronomy and cosmology, the three main causes of electromagnetic redshift are # The radiation travels between objects which are moving apart (" relativistic" redshift, an example of the relativistic Doppler effect) #The radiation travels towards an object in a weaker gravitational potential, i.e. towards an object in less strongly curved (flatter) spacetime (gravitational redshift) #The radiation travels through expanding space (cosmological redshift). The observation that all sufficiently distant light sources show redshift corresponding to their distance from Earth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |