|
Bafilomycin A1
The bafilomycins are a family of macrolide antibiotics produced from a variety of ''Streptomycetes''. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death. Bafilomycin A1 specifically targets the vacuolar-type H+ -ATPase (V-ATPase) enzyme, a membrane-spanning proton pump that acidifies either the extracellular environment or intracellular organelles such as the lysosome. At higher micromolar concentrations, bafilomycin A1 also acts on P-type ATPases, which have a phosphorylated transitional state. Bafilomycin A1 serves as an important tool compound in many ''in vitro'' researc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fermentek
Fermentek Ltd. is a biotechnological company in the Atarot industrial zone of Jerusalem, Israel. It specializes in the research, development and manufacture of biologically active, natural products isolated from microorganisms as well as from other natural sources such as plants and algae. The main microorganisms used are nonpathogenic actinomycetes, Nocardia and Streptomycetes. The fungi used are: Penicillium, Aspergillus, Fusarium and the like. None of these is a human pathogen. Fermentek does not sell to individuals. Most of its products are marketed through major international distributors specializing in chemicals, under their own brand names. Nevertheless, Fermentek has specific impact on the biochemical market, especially in the field of mycotoxins. Mycotoxins are toxic compounds produced by molds in human food and farm animal feeds, thus being economically important factors. Fermentek manufactures an extensive line of pure mycotoxins used as standards in food analysis. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bafilomycins Chemical Structures
The bafilomycins are a family of macrolide antibiotics produced from a variety of '' Streptomycetes''. Their chemical structure is defined by a 16-membered lactone ring scaffold. Bafilomycins exhibit a wide range of biological activity, including anti-tumor, anti-parasitic, immunosuppressant and anti-fungal activity. The most used bafilomycin is bafilomycin A1, a potent inhibitor of cellular autophagy. Bafilomycins have also been found to act as ionophores, transporting potassium K+ across biological membranes and leading to mitochondrial damage and cell death. Bafilomycin A1 specifically targets the vacuolar-type H+ -ATPase (V-ATPase) enzyme, a membrane-spanning proton pump that acidifies either the extracellular environment or intracellular organelles such as the lysosome. At higher micromolar concentrations, bafilomycin A1 also acts on P-type ATPases, which have a phosphorylated transitional state. Bafilomycin A1 serves as an important tool compound in many ''in vitro'' res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
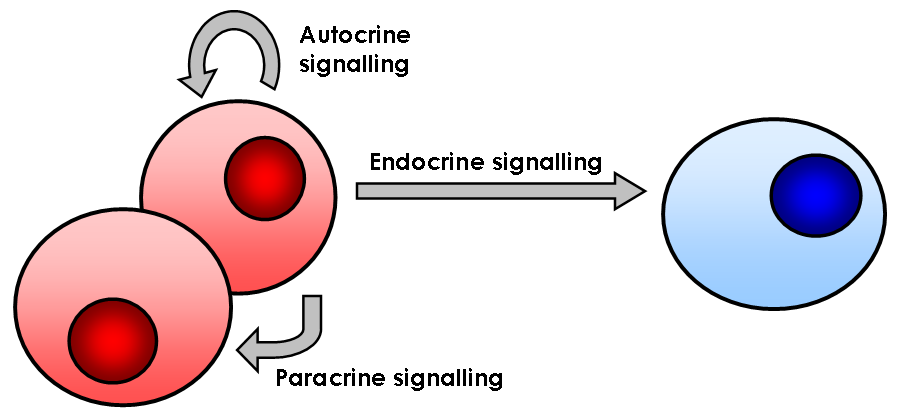
Cell Signaling
In biology, cell signaling (cell signalling in British English) or cell communication is the ability of a cell to receive, process, and transmit signals with its environment and with itself. Cell signaling is a fundamental property of all cellular life in prokaryotes and eukaryotes. Signals that originate from outside a cell (or extracellular signals) can be physical agents like mechanical pressure, voltage, temperature, light, or chemical signals (e.g., small molecules, peptides, or gas). Cell signaling can occur over short or long distances, and as a result can be classified as autocrine, juxtacrine, intracrine, paracrine, or endocrine. Signaling molecules can be synthesized from various biosynthetic pathways and released through passive or active transports, or even from cell damage. Receptors play a key role in cell signaling as they are able to detect chemical signals or physical stimuli. Receptors are generally proteins located on the cell surface or within the interio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Degradation
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endocytosis
Endocytosis is a cellular process in which substances are brought into the cell. The material to be internalized is surrounded by an area of cell membrane, which then buds off inside the cell to form a vesicle containing the ingested material. Endocytosis includes pinocytosis (cell drinking) and phagocytosis (cell eating). It is a form of active transport. History The term was proposed by De Duve in 1963. Phagocytosis was discovered by Élie Metchnikoff in 1882. Pathways Endocytosis pathways can be subdivided into four categories: namely, receptor-mediated endocytosis (also known as clathrin-mediated endocytosis), caveolae, pinocytosis, and phagocytosis Phagocytosis () is the process by which a cell uses its plasma membrane to engulf a large particle (≥ 0.5 μm), giving rise to an internal compartment called the phagosome. It is one type of endocytosis. A cell that performs phagocytosis is .... *Clathrin-mediated endocytosis is mediated by the production of smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osteoclast
An osteoclast () is a type of bone cell that breaks down bone tissue. This function is critical in the maintenance, repair, and remodeling of bones of the vertebral skeleton. The osteoclast disassembles and digests the composite of hydrated protein and mineral at a molecular level by secreting acid and a collagenase, a process known as ''bone resorption''. This process also helps regulate the level of blood calcium. Osteoclasts are found on those surfaces of bone that are undergoing resorption. On such surfaces, the osteoclasts are seen to be located in shallow depressions called ''resorption bays (Howship's lacunae)''. The resorption bays are created by the erosive action of osteoclasts on the underlying bone. The border of the lower part of an osteoclast exhibits finger-like processes due to the presence of deep infoldings of the cell membrane; this border is called ''ruffled border''. The ruffled border lies in contact with the bone surface within a resorption bay. The periph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endosome
Endosomes are a collection of intracellular sorting organelles in eukaryotic cells. They are parts of endocytic membrane transport pathway originating from the trans Golgi network. Molecules or ligands internalized from the plasma membrane can follow this pathway all the way to lysosomes for degradation or can be recycled back to the cell membrane in the endocytic cycle. Molecules are also transported to endosomes from the trans Golgi network and either continue to lysosomes or recycle back to the Golgi apparatus. Endosomes can be classified as early, sorting, or late depending on their stage post internalization. Endosomes represent a major sorting compartment of the endomembrane system in cells. Function Endosomes provide an environment for material to be sorted before it reaches the degradative lysosome. For example, low-density lipoprotein (LDL) is taken into the cell by binding to the LDL receptor at the cell surface. Upon reaching early endosomes, the LDL dissociates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
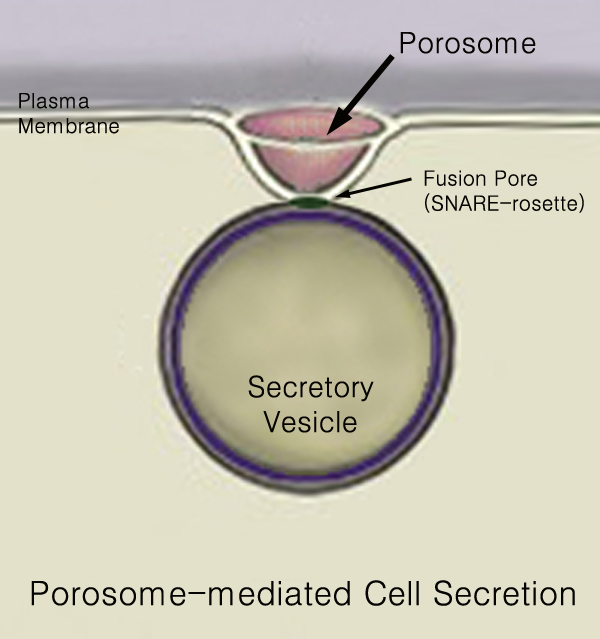
Secretory Vesicles
440px Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mechanism of cell secretion is via secretory portals at the plasma membrane called porosomes. Porosomes are permanent cup-shaped lipoprotein structures embedded in the cell membrane, where secretory vesicles transiently dock and fuse to release intra-vesicular contents from the cell. Secretion in bacterial species means the transport or translocation of effector molecules for example: proteins, enzymes or toxins (such as cholera toxin in pathogenic bacteria e.g. ''Vibrio cholerae'') from across the interior (cytoplasm or cytosol) of a bacterial cell to its exterior. Secretion is a very important mechanism in bacterial functioning and operation in their natural surrounding environment for adaptation and survival. In eukaryotic cells Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
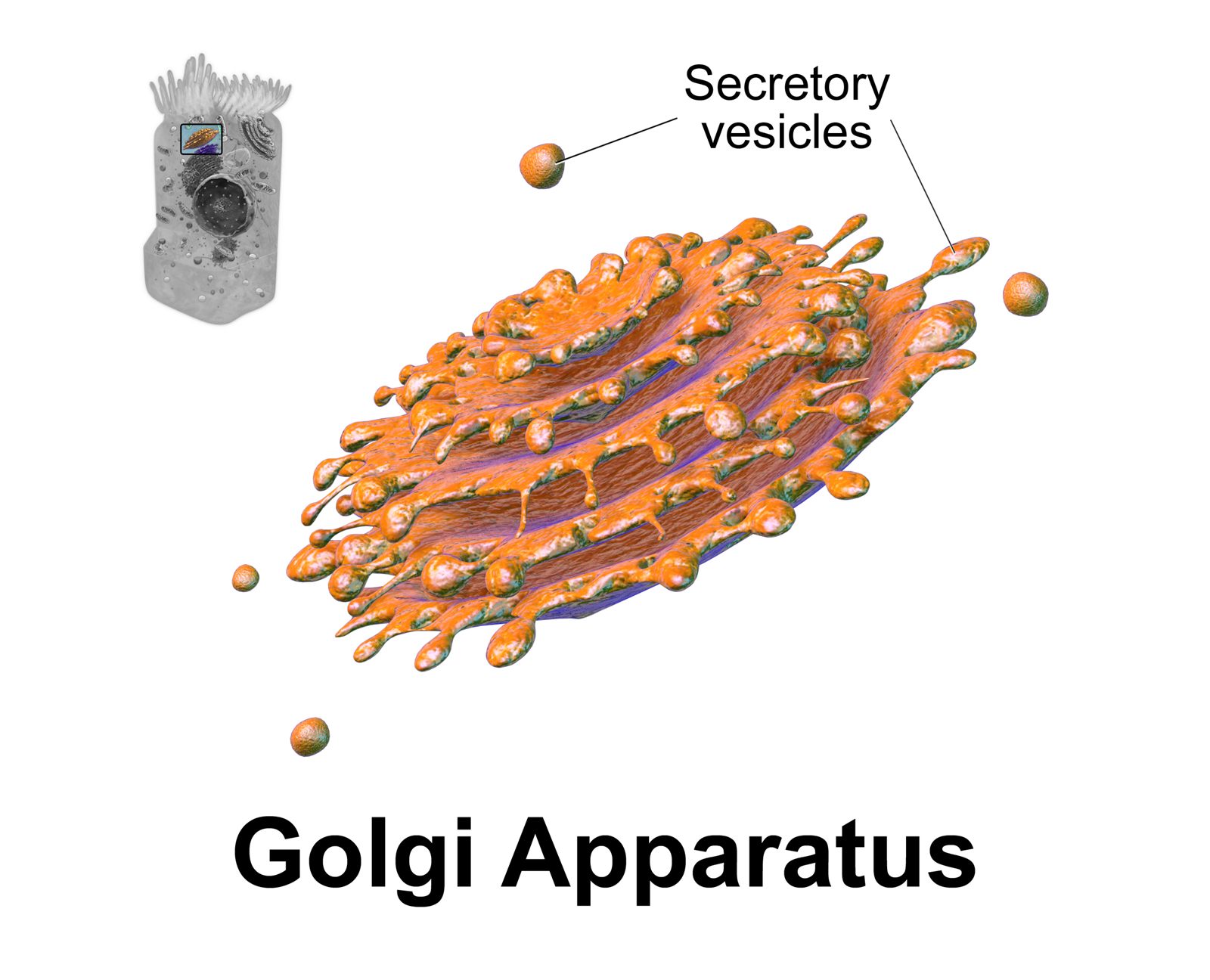
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clathrin-coated Vesicles
Clathrin is a protein that plays a major role in the formation of coated vesicles. Clathrin was first isolated and named by Barbara Pearse in 1976. It forms a triskelion shape composed of three clathrin heavy chains and three light chains. When the triskelia interact they form a polyhedral lattice that surrounds the vesicle, hence the protein's name, which is derived from the Latin ''clathrum'' meaning lattice. Coat-proteins, like clathrin, are used to build small vesicles in order to transport molecules within cells. The endocytosis and exocytosis of vesicles allows cells to communicate, to transfer nutrients, to import signaling receptors, to mediate an immune response after sampling the extracellular world, and to clean up the cell debris left by tissue inflammation. The endocytic pathway can be hijacked by viruses and other pathogens in order to gain entry to the cell during infection. Structure The clathrin triskelion is composed of three clathrin heavy chains int ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structure ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |