|
Azobisisobutyronitrile
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula CH3)2C(CN)sub>2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator. As an azo initiator, radicals resulting from AIBN have multiple benefits over common organic peroxides. For example, they do not have oxygenated byproducts or much yellow discoloration. Additionally, they do not cause too much grafting and therefore are often used when making adhesives, acrylic fibers, detergents, etc. Mechanism of decomposition In its most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals: : Because azobisisobutyronitrile readily gives off free radicals, it is often used as a radical initiator. This happens at temperatures above 40 °C, but in experiments is more commonly done at temperatures between 66 °C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylsuccinonitrile
Tetramethylsuccinonitrile or TMSN is an organic compound with the formula (C(CH3)2CN)2, classified as a di nitrile. It is a colorless and odorless solid. TMSN is the byproduct from the use of some radical initiators used in polymer manufacture. TMSN is derived from 2,2'-azobis-isobutyronitrile: :(NC(CH3)2CN)2 → (C(CH3)2CN)2 + N2 AIBN is a common radical initiator in the manufacture of polyvinyl chloride polymers. Safety considerations Because PVC is pervasive and can contain TMSN, the safety aspects of this dinitrile has generated interest. Symptoms of large or short exposure to this substance include convulsions, dizziness, headache, nausea, vomiting or even unconsciousness, hence affects central nervous system. In regards to occupational exposures, the Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency respo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical Initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical initiators are utilized in industrial processes such as polymer synthesis. Typical examples are molecules with a nitrogen-halogen bond, azo compounds, and organic and inorganic peroxides. Main types of initiation reaction *Halogens undergo homolytic fission relatively easily. Chlorine, for example, gives two chlorine radicals (Cl•) by irradiation with ultraviolet light. This process is used for chlorination of alkanes. *Azo compounds (R- N=N-R') can be the precursor of two carbon-centered radicals (R• and R'•) and nitrogen gas upon heating and/or by irradiation. For example, AIBN and ABCN yield isobutyronitrile and cyclohexanecarbonitrile radicals, respectively. : *Organic peroxides each have a peroxide bond (- O-O-), which is readi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacrylic Acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deprotonated derivatives thereof are known and of commercial value. In a water solution at neutral pH, PAA is an anionic polymer, i.e., many of the side chains of PAA lose their protons and acquire a negative charge. Partially or wholly deprotonated PAAs are polyelectrolytes, with the ability to absorb and retain water and swell to many times their original volume. These properties – acid-base and water-attracting – are the bases of many applications. = Synthesis = PAA is produced by free radical polymerization. Initiators include potassium persulfate and AIBN. Polyacrylic acid is a polyolefin. It can be viewed as polyethylene with carboxylic acid (CO2H) substituents on alternating carbons. Owing to these groups, alternating carbon atoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm. History The compound was first isolated in 1837 through a distillation of pine oil by the Polish chemist Filip Walter, who named it ''rétinnaphte''. In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree ''Myroxylon balsamum''), which Deville recognized as similar to Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzoyl Peroxide
Benzoyl peroxide is a chemical compound (specifically, an organic peroxide) with structural formula , often abbreviated as (BzO)2. In terms of its structure, the molecule can be described as two benzoyl (, Bz) groups connected by a peroxide (). It is a white granular solid with a faint odour of benzaldehyde, poorly soluble in water but soluble in acetone, ethanol, and many other organic solvents. Benzoyl peroxide is an oxidizer, but it is principally used as in the production of polymers. Benzoyl peroxide is mainly used in production of plastics and for bleaching flour, hair, plastics and textiles. Synthesis, structure, and chemical reactivity The original 1858 synthesis by Liebig reacted benzoyl chloride with barium peroxide, a reaction described by the following idealized equation: : Benzoyl peroxide is usually prepared by treating hydrogen peroxide with benzoyl chloride under alkaline conditions. : As in many other peroxides, the C-O-O-C linkage has a dihedral angle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Production Of AIBN
Production may refer to: Economics and business * Production (economics) * Production, the act of manufacturing goods * Production, in the outline of industrial organization, the act of making products (goods and services) * Production as a statistic, gross domestic product * Production line Arts, entertainment, and media Motion pictures * Production, film distributor of a company * Production, Filmmaking#Production, phase of filmmaking * Production, video production Other uses in arts, entertainment, and media * Production (album), ''Production'' (album), by Mirwais, 2000 * Production, magic (illusion)#Categories of effects, category of illusory magic trick * Production, Video game development#Production, phase of video games development * Production, Record producer's role * Production, Theatre, theatrical performance Science and technology * Production, Deployment environment#Production, deployment environment where changes go "live" and users interact with it * Production (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ABCN
1,1′-Azobis(cyclohexanecarbonitrile) or ACHN is a radical initiator. The molecular formula is NCC6H10N=NC6H10CN. It is a white solid that is soluble in aromatic solvents. ACHN has a 10-hour half-life in toluene Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) a ... at 88 °C. See also * Azobisisobutylonitrile (AIBN) is another commonly used free radical initiator References Azo compounds Nitriles Radical initiators {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
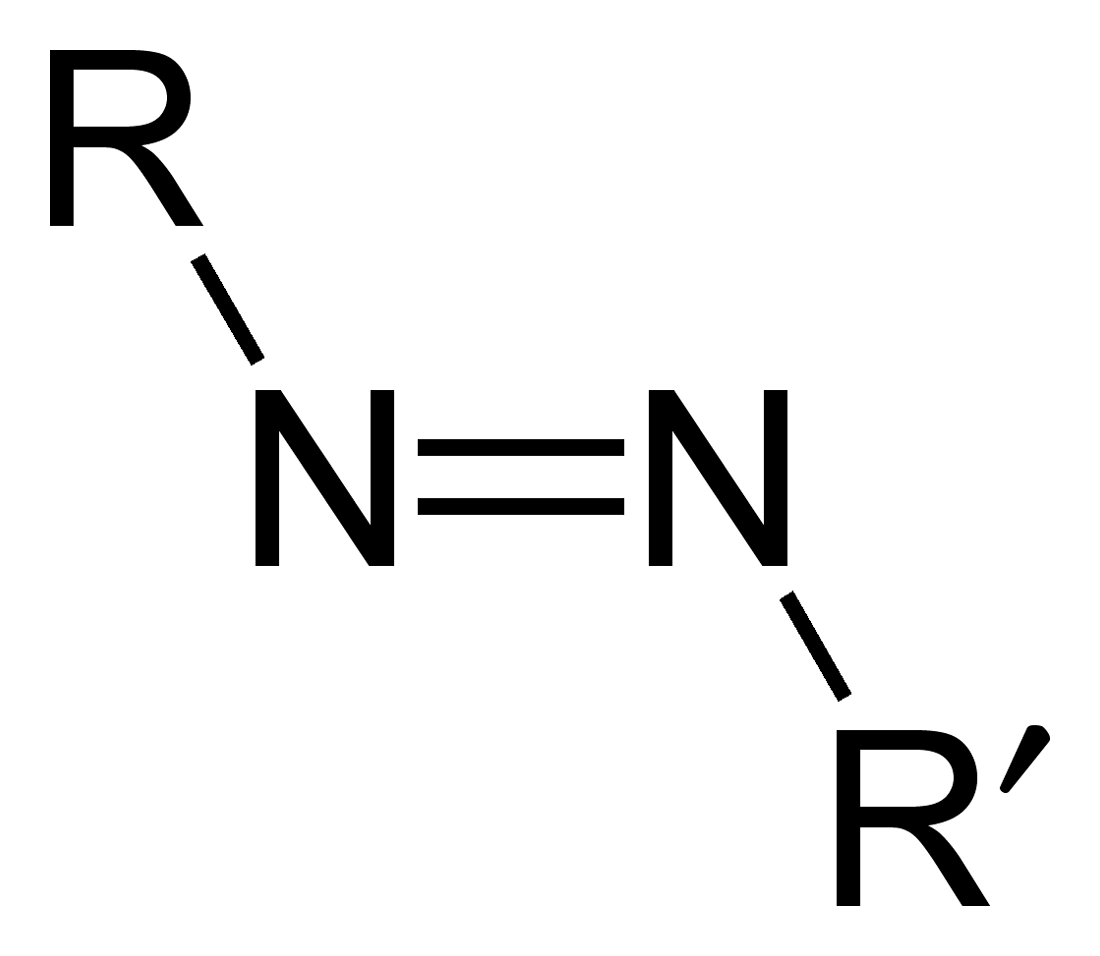
Azo Compound
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene." The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the ''trans'' isomer, but upon illumination, converts to the ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic substitution reaction where an aryl diazonium cation is attacked by another aryl ring, especially those substituted with electron-donating groups: :ArN2+ + Ar'H -> ArN=NAr' + H+ Since diazoniu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine hydrate (). Hydrazine is mainly used as a foaming agent in preparing polymer foams, but applications also include its uses as a precursor to polymerization catalysts, pharmaceuticals, and agrochemicals, as well as a long-term storable propellant for in-space spacecraft propulsion. Additionally, hydrazine is used in various rocket fuels and to prepare the gas precursors used in air bags. Hydrazine is used within both nuclear and conventional electrical power plant steam cycles as an oxygen scavenger to control concentrations of dissolved oxygen in an effort to reduce corrosion. the world hydrazine hydrate market amounted to $350 million. About two million tons of hydrazine hydrate were used in foam blowing agents in 2015. Hydrazines r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetone Cyanohydrin
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic. Preparation In the laboratory, this compound may be prepared by treating sodium cyanide with acetone, followed by acidification: : Considering the high toxicity of acetone cyanohydrin, a lab scale production has been developed using a microreactor-scale flow chemistry to avoid needing to manufacture and store large quantities of the reagent. Alternatively, a simplified procedure involves the action of sodium or potassium cyanide on the sodium bisulfite adduct of acetone prepared ''in situ''. This gives a less pure product, one that is nonetheless suitable for most syntheses. Reactions Acetone cyanohydrin is an intermediate en route to methyl methacrylate. Treat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_Reaction.png)

.png)