|
Asymmetric Ester Hydrolysis With Pig-liver Esterase
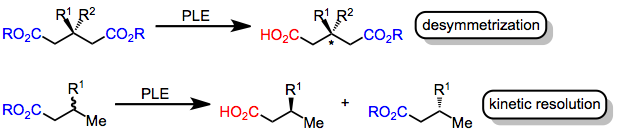
Asymmetric ester hydrolysis with pig liver esterase is the enantioselective synthesis, enantioselective conversion of an ester to a carboxylic acid through the action of the enzyme pig liver esterase (EC 3.1.1.1). Asymmetric ester hydrolysis involves the selective reaction of one of a pair of either topicity#Enantiotopic, enantiotopic (within the same molecule and related by a Reflection symmetry, symmetry plane of the molecule) or Chirality (chemistry), enantiomorphic (in enantiomeric molecules and related as mirror images) ester groups. Introduction Enzymes, which are composed of chiral amino acids, catalyze chemical reactions with high stereoselectivity. Specifically, esterase enzymes catalyze the hydrolysis of esters to carboxylic acids. This transformation may be rendered asymmetry, asymmetric if two enantiotopic ester groups exist in the substrate or if a racemic mixture of chiral esters is used. In the former case (desymmetrization), the chiral environment of the enzyme active ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantioselective Synthesis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecule and which produces the stereoisomeric (enantiomeric or diastereomeric) products in unequal amounts." Put more simply: it is the synthesis of a compound by a method that favors the formation of a specific enantiomer or diastereomer. Enantiomers are stereoisomers that have opposite configurations at every chiral center. Diastereomers are stereoisomers that differ at one or more chiral centers. Enantioselective synthesis is a key process in modern chemistry and is particularly important in the field of pharmaceuticals, as the different enantiomers or diastereomers of a molecule often have different biological activity. Overview Many of the building blocks of biological systems such as sugars and amino acids are produced exclusively as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diastereomer
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter, they are epimers. Each stereocenter gives rise to two different configurations and thus typically increases the number of stereoisomers by a factor of two. Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, excluding the opposing enantiomer). Diastereomers h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethyl Malonate
Dimethyl malonate is a diester derivative of malonic acid. It is a common reagent for organic synthesis used, for example, as a precursor for barbituric acid. It is also used in the malonic ester synthesis. It can be synthesized from dimethoxymethane and carbon monoxide. ::\mathrm Dimethyl malonate is used extensively in the fragrance industry as a raw material in the synthesis of jasmonates. For example, methyl dihydrojasmonate is synthesized from cyclopentanone, pentanal and dimethyl malonate. Hedione is used in almost all fine fragrances and is found in Christian Dior's ''Eau Sauvage'' and "Diorella", Hermes' "Voyage d'Hermes Parfum", Calvin Klein's "CKOne", Chanel's "Chanel No. 19", and Mark Jacob's "Blush", among others. As of 2009, Hedione was Firmenich's top selling compound by volume. Hebei Chengxin is the world's largest producer of dimethyl malonate by volume and uses a chloroacetic acid/sodium cyanide process developed in the 1940s.Stoesser, WC. "Preparation of malonic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glutarates
Glutaric acid is the organic compound with the formula C3H6(COOH)2 . Although the related "linear" dicarboxylic acids adipic and succinic acids are water-soluble only to a few percent at room temperature, the water-solubility of glutaric acid is over 50% (w/w). Biochemistry Glutaric acid is naturally produced in the body during the metabolism of some amino acids, including lysine and tryptophan. Defects in this metabolic pathway can lead to a disorder called glutaric aciduria, where toxic byproducts build up and can cause severe encephalopathy. Production Glutaric acid can be prepared by the ring-opening of butyrolactone with potassium cyanide to give the mixed potassium carboxylate-nitrile that is hydrolyzed to the diacid. Alternatively hydrolysis, followed by oxidation of dihydropyran gives glutaric acid. It can also be prepared from reacting 1,3-dibromopropane with sodium or potassium cyanide to obtain the dinitrile, followed by hydrolysis. Uses *1,5-Pentanediol, a co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object. An object or a system is ''chiral'' if it is distinguishable from its mirror image; that is, it cannot be superimposed onto it. Conversely, a mirror image of an ''achiral'' object, such as a sphere, cannot be distinguished from the object. A chiral object and its mirror image are called ''enantiomorphs'' (Greek, "opposite forms") or, when referring to molecules, '' enantiomers''. A non-chiral object is called ''achiral'' (sometimes also ''amphichiral'') and can be superposed on its mirror image. The term was first used by Lord Kelvin in 1893 in the second Robert Boyle Lecture at the Oxford University Junior Scientific Club which was published in 1894: Human hands are perhaps the most recognized example of chirality. The left hand is a non-superimposable mirror image of the right hand; no matter ho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |