Asymmetric Ester Hydrolysis With Pig-liver Esterase on:
[Wikipedia]
[Google]
[Amazon]
Asymmetric ester hydrolysis with pig liver esterase is the enantioselective conversion of an

 3-Alkyl glutarates with small alkyl substituents are hydrolyzed to the (''R'')-monoester; however, when a large alkyl substituent is present, the (''S'')-monoester forms. This switch in enantioselectivity is accurately predicted by the active site model given above.
''(4)''
3-Alkyl glutarates with small alkyl substituents are hydrolyzed to the (''R'')-monoester; however, when a large alkyl substituent is present, the (''S'')-monoester forms. This switch in enantioselectivity is accurately predicted by the active site model given above.
''(4)'' An opposite trend is observed in desymmetrizing hydrolyses of 2-methyl malonates, which afford the (''S'') enantiomer when the other substituent on C-2 is small, and the (''R'') enantiomer when the other C-2 substituent is large.
''(5)''
An opposite trend is observed in desymmetrizing hydrolyses of 2-methyl malonates, which afford the (''S'') enantiomer when the other substituent on C-2 is small, and the (''R'') enantiomer when the other C-2 substituent is large.
''(5)'' A number of ''meso'' diesters other than the substrates described above may be hydrolyzed by PLE with high enantioselectivity. Cyclic ''meso'' diesters tend to be hydrolyzed more selectively than acyclic diesters.Mohr, P.; Waespe-Sarevi, N.; Tamm, C.; Gawronska, K.; Gawronski, J. ''Helv. Chim. Acta'' 1983, ''66'', 2501. The predominant enantiomer of product depends on ring size.
''(6)''
A number of ''meso'' diesters other than the substrates described above may be hydrolyzed by PLE with high enantioselectivity. Cyclic ''meso'' diesters tend to be hydrolyzed more selectively than acyclic diesters.Mohr, P.; Waespe-Sarevi, N.; Tamm, C.; Gawronska, K.; Gawronski, J. ''Helv. Chim. Acta'' 1983, ''66'', 2501. The predominant enantiomer of product depends on ring size.
''(6)'' 7-Oxabicyclo .2.1eptane-2,3-dicarboxylates are an interesting class of diesters that are hydrolyzed by PLE with high enantioselectivity. These substrates have been used for the enantioselective construction of biologically relevant sugars (see Synthetic Applications below).
''(7)''
7-Oxabicyclo .2.1eptane-2,3-dicarboxylates are an interesting class of diesters that are hydrolyzed by PLE with high enantioselectivity. These substrates have been used for the enantioselective construction of biologically relevant sugars (see Synthetic Applications below).
''(7)'' Racemic mixtures of all of the substrates described above, as well as additional chiral diesters (such as the epoxy ester in equation (8)), may be resolved using PLE for kinetic resolution. A significant disadvantage of kinetic resolution is a maximum yield of hydrolyzed product of 50%. However, if rapid racemization is occurring alongside hydrolysis (an example of
Racemic mixtures of all of the substrates described above, as well as additional chiral diesters (such as the epoxy ester in equation (8)), may be resolved using PLE for kinetic resolution. A significant disadvantage of kinetic resolution is a maximum yield of hydrolyzed product of 50%. However, if rapid racemization is occurring alongside hydrolysis (an example of  Esterase enzymes may also be used for hydrolysis of base-sensitive monoesters. PLE has been applied to the synthesis of prostaglandins for the selective hydrolysis of the ester without destruction of the β-hydroxy ketone moiety.
''(9)''
Esterase enzymes may also be used for hydrolysis of base-sensitive monoesters. PLE has been applied to the synthesis of prostaglandins for the selective hydrolysis of the ester without destruction of the β-hydroxy ketone moiety.
''(9)''
 Enantioselective hydrolysis of a conjugated diester followed by ozonolysis affords the skeleton of ribose. The resulting sugars are then carried on for the synthesis of nucleosides.
''(11)''
Enantioselective hydrolysis of a conjugated diester followed by ozonolysis affords the skeleton of ribose. The resulting sugars are then carried on for the synthesis of nucleosides.
''(11)'' L-α-Methyldopa may be rapidly synthesized from an achiral malonate through a sequence beginning with desymmetrization. Subsequent chemoselective transformations convert the carboxylic acid to an amine.
''(12)''
L-α-Methyldopa may be rapidly synthesized from an achiral malonate through a sequence beginning with desymmetrization. Subsequent chemoselective transformations convert the carboxylic acid to an amine.
''(12)''

ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ar ...
to a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
through the action of the enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. A ...
pig liver esterase
An esterase is a hydrolase enzyme that splits esters into an acid and an alcohol in a chemical reaction with water called hydrolysis.
A wide range of different esterases exist that differ in their substrate specificity, their protein structure, ...
(EC 3.1.1.1). Asymmetric ester hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
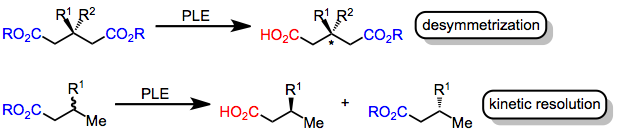
involves the selective reaction of one of a pair of either enantiotopic In stereochemistry, topicity is the stereochemical relationship between substituents and the structure to which they are attached. Depending on the relationship, such groups can be ''heterotopic'', ''homotopic'', ''enantiotopic'', or ''diastereotopi ...
(within the same molecule and related by a symmetry plane of the molecule) or enantiomorphic
In chemistry, a molecule or ion is called chiral () if it cannot be superposed on its mirror image by any combination of rotations, translations, and some conformational changes. This geometric property is called chirality (). The terms are d ...
(in enantiomeric molecules and related as mirror images) ester groups.
Introduction
Enzymes, which are composed ofchiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from ...
amino acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha am ...
, catalyze chemical reactions with high stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation of ...
. Specifically, esterase enzymes catalyze the hydrolysis of esters to carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic ...
s. This transformation may be rendered asymmetric if two enantiotopic ester groups exist in the substrate or if a racemic mixture
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
of chiral esters is used. In the former case (desymmetrization
Desymmetrization in stereochemistry is the modification of a molecule that results in the loss of one or more symmetry elements. A common application of this class of reactions involves the introduction of chirality.Willis, Michael C. "Enantiose ...
), the chiral environment of the enzyme active site leads to selective hydrolysis of the ester that is closer to the catalytically active serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form un ...
residue when the substrate is bound to the enzyme. In the latter case (kinetic resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resu ...
), one of the enantiomers is hydrolyzed faster than the other, leading to an excess of hydrolyzed product from one enantiomer. Both strategies rely on the fact that the transition states for hydrolysis of enantiotopic or enantiomorphic ester groups by the chiral enzyme are diastereomeric
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have di ...
.
Pig liver esterase (PLE) is a widely used enzyme for asymmetric ester hydrolysis. Although it was originally used for the desymmetrizing hydrolysis of glutarate esters,Cohen, S.; Khedouri, E. ''J. Am. Chem. Soc.'' 1961, ''83'', 1093. PLE also hydrolyzes malonate
The conjugate acids are in :Carboxylic acids.
{{Commons category, Carboxylate ions, Carboxylate anions
Carbon compounds
Oxyanions ...
s, cyclic diesters, monoesters, and other substrates. Active site models have been advanced to explain the selectivity of PLE.
''(1)''
Mechanism and Stereochemistry
Prevailing Mechanism
The active site of PLE facilitates both substrate binding and hydrolysis. A key serine residue in the active site promotes hydrolysis, but the substrate must present an ester group to this residue after binding to the enzyme active site for hydrolysis to take place. Whether the substrate is able to present an ester group to the catalytic serine residue depends on its bound conformation in the active site, which is dictated by amino acid side-chains in the active site. Thus, active site models of PLE have been advanced with the goal of predicting from the structure of the substrate which of two enantiotopic ester groups will be hydrolyzed (or whether hydrolysis is likely to occur at all). A simple model for the binding conformation of an ester in the active site of PLE is shown below. This model accurately predicts the configuration of hydrolyzed glutarates and similar substrates. ''(2)''
Scope and Limitations
Although the substrate scope of PLE is broad, enantioselectivity varies as a function of the structure of the substrate. This section describes substrates that are hydrolyzed by PLE with the highest enantioselectivity, as well as sensitive substrates that may be hydrolyzed toachiral
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
carboxylic acids in high yield without side reactions.
Glutarates were the first substrates to be hydrolyzed with PLE in high enantioselectivity. Although yields are moderate, enantioselectivity is extremely high.Huang, F.-C.; Lee, L. F. H.; Mittal, R. S. D.; Ravikumar, P. R.; Chan, J. A.; Sih, C. J.; Capsi, E.; Eck, C. R. ''J. Am. Chem. Soc.'' 1975, ''97'', 4144.
''(3)'' 3-Alkyl glutarates with small alkyl substituents are hydrolyzed to the (''R'')-monoester; however, when a large alkyl substituent is present, the (''S'')-monoester forms. This switch in enantioselectivity is accurately predicted by the active site model given above.
''(4)''
3-Alkyl glutarates with small alkyl substituents are hydrolyzed to the (''R'')-monoester; however, when a large alkyl substituent is present, the (''S'')-monoester forms. This switch in enantioselectivity is accurately predicted by the active site model given above.
''(4)'' An opposite trend is observed in desymmetrizing hydrolyses of 2-methyl malonates, which afford the (''S'') enantiomer when the other substituent on C-2 is small, and the (''R'') enantiomer when the other C-2 substituent is large.
''(5)''
An opposite trend is observed in desymmetrizing hydrolyses of 2-methyl malonates, which afford the (''S'') enantiomer when the other substituent on C-2 is small, and the (''R'') enantiomer when the other C-2 substituent is large.
''(5)'' A number of ''meso'' diesters other than the substrates described above may be hydrolyzed by PLE with high enantioselectivity. Cyclic ''meso'' diesters tend to be hydrolyzed more selectively than acyclic diesters.Mohr, P.; Waespe-Sarevi, N.; Tamm, C.; Gawronska, K.; Gawronski, J. ''Helv. Chim. Acta'' 1983, ''66'', 2501. The predominant enantiomer of product depends on ring size.
''(6)''
A number of ''meso'' diesters other than the substrates described above may be hydrolyzed by PLE with high enantioselectivity. Cyclic ''meso'' diesters tend to be hydrolyzed more selectively than acyclic diesters.Mohr, P.; Waespe-Sarevi, N.; Tamm, C.; Gawronska, K.; Gawronski, J. ''Helv. Chim. Acta'' 1983, ''66'', 2501. The predominant enantiomer of product depends on ring size.
''(6)'' 7-Oxabicyclo .2.1eptane-2,3-dicarboxylates are an interesting class of diesters that are hydrolyzed by PLE with high enantioselectivity. These substrates have been used for the enantioselective construction of biologically relevant sugars (see Synthetic Applications below).
''(7)''
7-Oxabicyclo .2.1eptane-2,3-dicarboxylates are an interesting class of diesters that are hydrolyzed by PLE with high enantioselectivity. These substrates have been used for the enantioselective construction of biologically relevant sugars (see Synthetic Applications below).
''(7)'' Racemic mixtures of all of the substrates described above, as well as additional chiral diesters (such as the epoxy ester in equation (8)), may be resolved using PLE for kinetic resolution. A significant disadvantage of kinetic resolution is a maximum yield of hydrolyzed product of 50%. However, if rapid racemization is occurring alongside hydrolysis (an example of
Racemic mixtures of all of the substrates described above, as well as additional chiral diesters (such as the epoxy ester in equation (8)), may be resolved using PLE for kinetic resolution. A significant disadvantage of kinetic resolution is a maximum yield of hydrolyzed product of 50%. However, if rapid racemization is occurring alongside hydrolysis (an example of dynamic kinetic resolution
In organic chemistry, kinetic resolution is a means of differentiating two enantiomers in a racemic mixture. In kinetic resolution, two enantiomers react with different reaction rates in a chemical reaction with a chiral catalyst or reagent, resul ...
), a maximum yield of 100% is possible.
''(8)'' Esterase enzymes may also be used for hydrolysis of base-sensitive monoesters. PLE has been applied to the synthesis of prostaglandins for the selective hydrolysis of the ester without destruction of the β-hydroxy ketone moiety.
''(9)''
Esterase enzymes may also be used for hydrolysis of base-sensitive monoesters. PLE has been applied to the synthesis of prostaglandins for the selective hydrolysis of the ester without destruction of the β-hydroxy ketone moiety.
''(9)''
Synthetic Applications
A number of synthetic targets possess hidden symmetry that may be discovered by applying a retrosynthetic "symmetrizing" transform. In the forward direction, this operation corresponds to a desymmetrization reaction. For instance, mevalonolactone may be synthesized rapidly from a symmetric diester via desymmetrizing hydrolysis, chemoselective reduction, and lactonization. Although the product itself is asymmetric, desymmetrization and functional group manipulations permit its synthesis from an achiral starting material. ''(10)'' Enantioselective hydrolysis of a conjugated diester followed by ozonolysis affords the skeleton of ribose. The resulting sugars are then carried on for the synthesis of nucleosides.
''(11)''
Enantioselective hydrolysis of a conjugated diester followed by ozonolysis affords the skeleton of ribose. The resulting sugars are then carried on for the synthesis of nucleosides.
''(11)'' L-α-Methyldopa may be rapidly synthesized from an achiral malonate through a sequence beginning with desymmetrization. Subsequent chemoselective transformations convert the carboxylic acid to an amine.
''(12)''
L-α-Methyldopa may be rapidly synthesized from an achiral malonate through a sequence beginning with desymmetrization. Subsequent chemoselective transformations convert the carboxylic acid to an amine.
''(12)''
Comparison with Other Methods
Other enzymes that may be used for asymmetric ester hydrolysis include electric eel acetylcholinesterase, chymotrypsin, andBaker's yeast
Baker's yeast is the common name for the strains of yeast commonly used in baking bread and other bakery products, serving as a leavening agent which causes the bread to rise (expand and become lighter and softer) by converting the fermentable ...
. The substrate scope of these enzymes differs from PLE, and in some cases they may provide hydrolyzed products in higher yield or enantioselectivity than PLE. Microorganisms
A microorganism, or microbe,, ''mikros'', "small") and ''organism'' from the el, ὀργανισμός, ''organismós'', "organism"). It is usually written as a single word but is sometimes hyphenated (''micro-organism''), especially in olde ...
may also be used for enantioselective hydrolysis; however, difficulties associated with the handling of microorganisms have made these methods unpopular for organic synthesis.
Nonenzymatic methods for the differentiation of enantiotopic groups employ chiral catalysts or auxiliaries. For instance, the introduction of a chiral leaving group on both carboxylic acid groups of a ''meso'' diacid leads to selective attack by an achiral nucleophile at one of the (now) diastereotopic carbonyl groups.Nagao, Y.; Ikeda, T.; Yagi, M.; Fujita, E.; Shiro, M. ''J. Am. Chem. Soc.'' 1982, ''104'', 2079.
''(13)''
Experimental Conditions and Procedure
Typical Conditions
Enzymatic reactions are limited by the need for aqueous solvent and near-neutral reaction conditions. PLE hydrolyses are typically carried out with a phosphate buffer to maintain the pH between 7 and 8. As solubility of the substrate in the aqueous medium is critical, a small amount of a polar organic co-solvent is sometimes added to the aqueous solution of the enzyme. Commercially available PLE is of sufficient purity for most applications.References
{{reflist, 2 Organic reactions