|
Arsine
Arsine (IUPAC name: arsane) is an inorganic compound with the formula As H3. This flammable, pyrophoric, and highly toxic pnictogen hydride gas is one of the simplest compounds of arsenic. Despite its lethality, it finds some applications in the semiconductor industry and for the synthesis of organoarsenic compounds. The term ''arsine'' is commonly used to describe a class of organoarsenic compounds of the formula AsH3ãxRx, where R = aryl or alkyl. For example, As(C6H5)3, called triphenylarsine, is referred to as "an arsine". General properties At its standard state, arsine is a colorless, denser-than-air gas that is slightly soluble in water (20% at 20 ô¯C) and in many organic solvents as well. Whereas arsine itself is odorless, owing to its oxidation by air, it is possible to smell a slight garlic or fish-like scent when the compound is present above 0.5 ppm. This compound is kinetically stable: at room temperature it decomposes only slowly. At temperatures of ca. 230&n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pnictogen Hydride
Pnictogen hydrides or hydrogen pnictides are binary compounds of hydrogen with pnictogen ( or ; from grc, üö§Ã¢äö°ü "to choke" and -gen, "generator") atoms (elements of group 15: nitrogen, phosphorus, arsenic, antimony, and bismuth) covalently bonded to hydrogen. Pnictogen trihydrides The simplest series has the chemical formula XH3 (less commonly H3X), with X representing any of the pnictogens. They take on the pyramidal structure (as opposed to the trigonal planar arrangement of the group 13 hydrides), and therefore are polar. These pnictogen trihydrides are generally increasingly unstable and poisonous with heavier elements. Like the simple hydrogen halides and chalcogenides, the pnictogen hydrides are water- soluble. Unlike other hydrides such as hydrogen sulfide and hydrogen fluoride, which form acidic aqueous solutions, ammonia dissolves in water to make ammonium hydroxide which is basic (by forming a hydroxide ion as opposed to hydronium). Phosphine is also water-sol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marsh Test
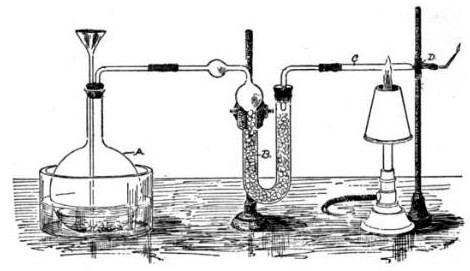
The Marsh test is a highly sensitive method in the detection of arsenic, especially useful in the field of forensic toxicology when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836. The method continued to be used, with improvements, in forensic toxicology until the 1970s. Arsenic, in the form of white arsenic trioxide , was a highly favored poison, being odourless, easily incorporated into food and drink, and before the advent of the Marsh test, untraceable in the body. In France, it came to be known as ' ("inheritance powder"). For the untrained, arsenic poisoning will have symptoms similar to cholera. Precursor methods The first breakthrough in the detection of arsenic poisoning was in 1775 when Carl Wilhelm Scheele discovered a way to change arsenic trioxide to garlic-smelling arsine gas (AsH3), by treating it with nitric acid (HNO3) and combining it with zinc. :As2O3 + 6 Zn + 12 HNO3 ã 2 AsH3 + 6 Zn(NO3)2 + 3 H2O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stibine
Stibine (IUPAC name: stibane) is a chemical compound with the formula SbH3. A pnictogen hydride, this colourless, highly toxic gas is the principal covalent hydride of antimony, and a heavy analogue of ammonia. The molecule is pyramidal with HãSbãH angles of 91.7ô¯ and SbãH distances of 170.7 pm (1.707 û ). This gas has an offensive smell like hydrogen sulfide (rotten eggs). Preparation SbH3 is generally prepared by the reaction of Sb3+ sources with Hã equivalents: :2 Sb2O3 + 3 LiAlH4 ã 4 SbH3 + 1.5 Li2O + 1.5 Al2O3 :4 SbCl3 + 3 NaBH4 ã 4 SbH3 + 3 NaCl + 3 BCl3 Alternatively, sources of Sb3− react with protonic reagents (even water) to also produce this unstable gas: :Na3Sb + 3 H2O ã SbH3 + 3 NaOH Properties The chemical properties of SbH3 resemble those for AsH3. Typical for a heavy hydride (e.g. AsH3, H2Te, SnH4), SbH3 is unstable with respect to its elements. The gas decomposes slowly at room temperature but rapidly at 200 ô¯C: ::2 SbH3 ã 3 H2 + 2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arsonium
The arsonium cation is a positively charged polyatomic ion with the chemical formula . An arsonium salt is a salt containing either the arsonium () cation, such as arsonium bromide () and arsonium iodide (), which can be synthesized by reacting arsine with hydrogen bromide Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room tempe ... or hydrogen iodide.MuûÝozãHernûÀndez, M. û. (2006). Arsenic: Inorganic Chemistry. ''Encyclopedia of Inorganic Chemistry''. pp 4. DOI: 10.1002/0470862106.ia013 Or more commonly, as organic derivative such as the quaternary arsonium salts (CAS: , hydrate form) and the zwitterionic compound arsenobetaine. References {{chem-stub Arsenic(ãIII) compounds Cations ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
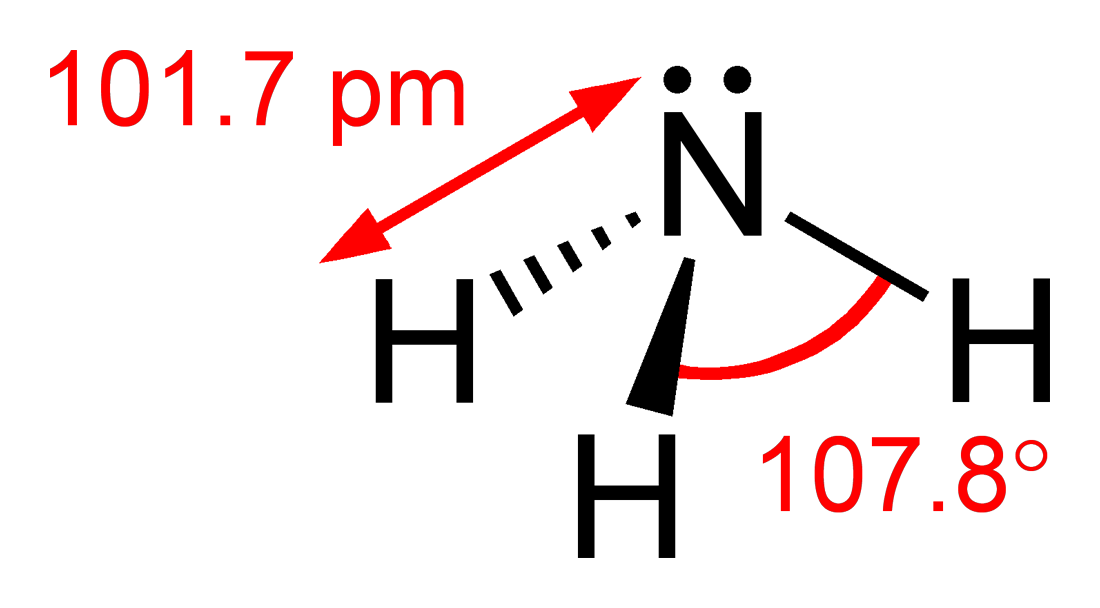
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in natureãboth terrestrially and in the outer planets of the Solar Systemãand in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting fish, due to the presence of substituted phosphine and diphosphane (). With traces of present, is spontaneously flammable in air ( pyrophoric), burning with a luminous flame. Phosphine is a highly toxic respiratory poison, and is immediately dangerous to life or health at 50 ppm. Phosphine has a trigonal pyramidal structure. Phosphines are compounds that include and the organophosphines, which are derived from by substituting one or more hydrogen atoms with organic groups. They have the general formula . Phosphanes are saturated phosphorus hydrides of the form , such as triphosphane. Phosphine, PH3, is the smallest of the phosphines and the smallest of the phosphanes. History Philippe Gengembre (1764ã1838), a student of Lavois ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuthine
Bismuthine (IUPAC name: bismuthane) is the chemical compound with the formula BiH3. As the heaviest analogue of ammonia (a pnictogen hydride), BiH3 is unstable, decomposing to bismuth metal well below 0 ô¯C. This compound adopts the expected pyramidal structure with HãBiãH angles of around 90ô¯. The term ''bismuthine'' may also refer to a member of the family of organobismuth(III) species having the general formula , where R is an organic substituent. For example, Bi(CH3)3 is ''trimethylbismuthine''. Preparation and properties BiH3 is prepared by the redistribution of methylbismuthine (BiH2Me):Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001.. :3 BiH2Me ã 2 BiH3 + BiMe3 The required BiH2Me, which is also thermally unstable, is generated by reduction of methylbismuth dichloride, BiCl2Me with LiAlH4. As suggested by the behavior of SbH3, BiH3 is unstable and decomposes to its constituent elements according to the following equatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Humidity
Humidity is the concentration of water vapor present in the air. Water vapor, the gaseous state of water, is generally invisible to the human eye. Humidity indicates the likelihood for precipitation, dew, or fog to be present. Humidity depends on the temperature and pressure of the system of interest. The same amount of water vapor results in higher relative humidity in cool air than warm air. A related parameter is the dew point. The amount of water vapor needed to achieve saturation increases as the temperature increases. As the temperature of a parcel of air decreases it will eventually reach the saturation point without adding or losing water mass. The amount of water vapor contained within a parcel of air can vary significantly. For example, a parcel of air near saturation may contain 28 g of water per cubic metre of air at , but only 8 g of water per cubic metre of air at . Three primary measurements of humidity are widely employed: absolute, relative, and specific. Ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Solvent
A solvent (s) (from the Latin '' solvé'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. The quantity of solute that can dissolve in a specific volume of solvent varies with temperature. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical synt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Garlic
Garlic (''Allium sativum'') is a species of bulbous flowering plant in the genus ''Allium''. Its close relatives include the onion, shallot, leek, chive, Allium fistulosum, Welsh onion and Allium chinense, Chinese onion. It is native to South Asia, Central Asia and northeastern Iran and has long been used as a seasoning worldwide, with a history of several thousand years of human consumption and use. It was known to ancient Egyptians and has been used as both a food flavoring and a traditional medicine. China produces 76% of the world's supply of garlic. Etymology The word ''garlic'' derives from Old English, ''garláac'', meaning ''gar'' (spear) and leek, as a 'spear-shaped leek'. Description ''Allium sativum'' is a perennial flowering plant growing from a bulb. It has a tall, erect flowering stem that grows up to . The leaf blade is flat, linear, solid, and approximately wide, with an acute apex. The plant may produce pink to purple flowers from July to September in the Nort ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts Per Million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are parts-per-million (ppm, ), parts-per-billion (ppb, ), parts-per-trillion (ppt, ) and parts-per-quadrillion (ppq, ). This notation is not part of the International System of Units (SI) system and its meaning is ambiguous. Overview Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. Therefore, it is common to equat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
û
ngstrûÑm
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ûËngstrûÑm is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is û , a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas û ngstrûÑm (1814ã1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals Lect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

-3D-balls.png)



.jpg)
