|
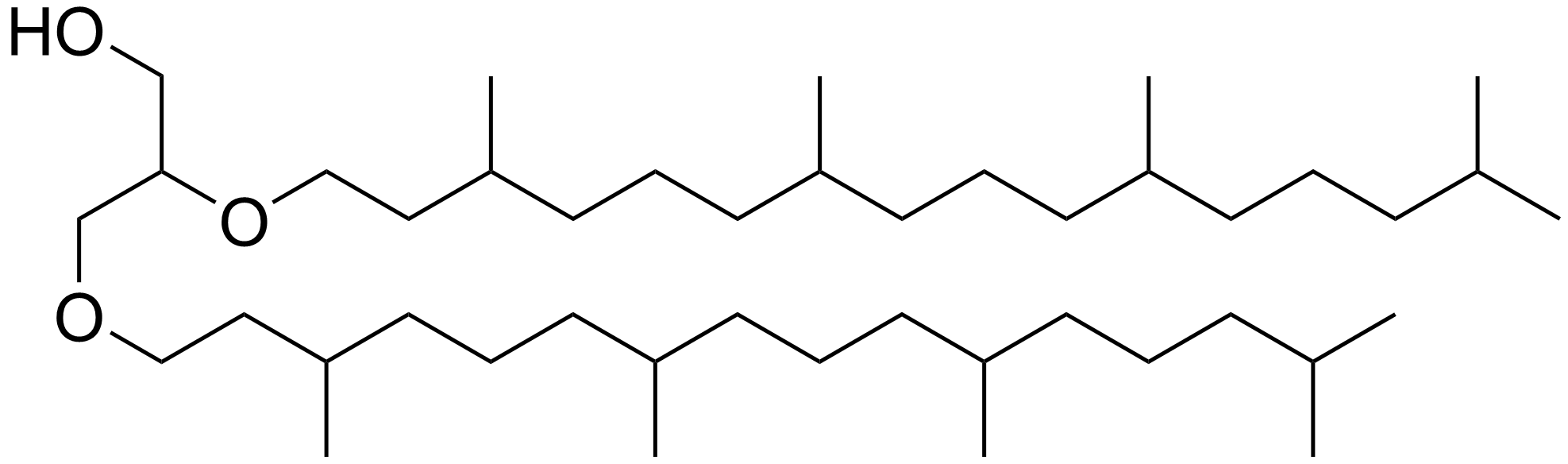
Archaeol
Archaeol is composed of two phytanyl chains linked to the sn-2 and sn-3 positions of glycerol. As its phosphate ester, it is a common component of the membranes of archaea. Structure and contrast with other lipids Archaeol is a diether. The 2,3-sn-glycerol structure and ether bond linkage are two key differences between lipids found in archaea vs those of bacteria and eukarya. The latter use 1,2-sn-glycerol, and mostly, ester bonds. Natural archaeol has 3R, 7R, 11R configurations for the three chiral centers in the isoprenoid chains. There are four structural variations, contributing to the complexity of the membrane lipids in function and properties. The two phytanyl chains can form a 36-member ring to yield macrocyclic archaeol. Hydroxylated archaeol has phytanyl chains hydroxylated at the first tertiary carbon atom, while sesterterpanyl archaeol have the phytanyl side chains with C25 sesterterpanyl chains, substituting at C2 of glycerol or at both carbons. Unsaturated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyarchaeol
Hydroxyarchaeol is a core lipid unique to archaea, similar to archaeol, with a hydroxide functional group at the carbon-3 position of one of its ether side chains. It is found exclusively in certain taxa of methanogenic archaea, and is a common biomarker for methanogenesis and methane-oxidation. Isotopic analysis of hydroxyarchaeol can be informative about the environment and substrates for methanogenesis. Discovery Hydroxyarchaeol was first identified by Dennis G. Sprott and colleagues in 1990 from ''Methanosaeta concilii'' by a combination of TLC, NMR and mass spectrometric analysis. Structure and function The lipid consists of a glycerol backbone with two C20 phytanyl ether chains attached, one of which has a hydroxyl (-OH) group attached at the C3 carbon. It is one of the major core lipids of methanogenic archaea alongside archaeol, forming the basis of their cell membrane. The two major forms are sn-2- and sn-3-hydroxyarchaeol, depending on if the hydroxyl group i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol Dialkyl Glycerol Tetraether
Glycerol dialkyl glycerol tetraether lipids (GDGTs) are a class of membrane lipids synthesized by archaea and some bacteria, making them useful biomarkers for these organisms in the geological record. Their presence, structure, and relative abundances in natural materials can be useful as proxies for temperature, terrestrial organic matter input, and soil pH for past periods in Earth history. Some structural forms of GDGT form the basis for the TEX86 paleothermometer. Isoprenoid GDGTs, now known to be synthesized by many archaeal classes, were first discovered in extremophilic archaea cultures. Branched GDGTs, likely synthesized by acidobacteriota, were first discovered in a natural Dutch peat sample in 2000. Chemical structure The two primary structural classes of GDGTs are isoprenoid (isoGDGT) and branched (brGDGT), which refer to differences in the carbon skeleton structures. Isoprenoid compounds are numbered -0 through -8, with the numeral representing the number of cyclop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Caldarchaeol
Caldarchaeol is a membrane-spanning lipid found in hyperthermophilic archaea. Membranes made up of caldarchaeol are more stable since the hydrophobic chains are linked together, allowing the microorganisms to withstand high temperatures. It is also known as dibiphytanyldiglycerol tetraether. Two glycerol units are linked together by two strains which consist of two phytanes linked together to form a linear chain of 32 carbon atoms (40 carbons including methyl sidechains). The configuration Configuration or configurations may refer to: Computing * Computer configuration or system configuration * Configuration file, a software file used to configure the initial settings for a computer program * Configurator, also known as choice boar ... of the macrocyclic tetraether has been determined by total synthesis of the C40-diol and comparison with a sample of obtained by degradation of natural tetraether. A synthesis of tetraether has also been carried out. Notes {{reflist Addi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytane
Phytane is the isoprenoid alkane formed when phytol, a constituent of chlorophyll, loses its hydroxyl group. When phytol loses one carbon atom, it yields pristane. Other sources of phytane and pristane have also been proposed than phytol. Pristane and phytane are common constituents in petroleum and have been used as proxies for depositional redox conditions, as well as for correlating oil and its source rock (i.e. elucidating where oil formed). In environmental studies, pristane and phytane are target compounds for investigating oil spills. Chemistry Phytane is a non-polar organic compound that is a clear and colorless liquid at room temperature. It is a head-to-tail linked regular isoprenoid with chemical formula C20H42. Phytane has many structural isomers. Among them, crocetane is a tail-to-tail linked isoprenoid and often co-elutes with phytane during gas chromatography (GC) due to its structural similarity. Phytane also has many stereoisomers because of its three st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Archaea
Archaea ( ; singular archaeon ) is a domain of single-celled organisms. These microorganisms lack cell nuclei and are therefore prokaryotes. Archaea were initially classified as bacteria, receiving the name archaebacteria (in the Archaebacteria kingdom), but this term has fallen out of use. Archaeal cells have unique properties separating them from the other two domains, Bacteria and Eukaryota. Archaea are further divided into multiple recognized phyla. Classification is difficult because most have not been isolated in a laboratory and have been detected only by their gene sequences in environmental samples. Archaea and bacteria are generally similar in size and shape, although a few archaea have very different shapes, such as the flat, square cells of '' Haloquadratum walsbyi''. Despite this morphological similarity to bacteria, archaea possess genes and several metabolic pathways that are more closely related to those of eukaryotes, notably for the enzymes invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycerol 1-phosphate
''sn''-Glycerol 1-phosphate is the conjugate base of a phosphoric ester of glycerol. It is a component of ether lipids, which are common for archaea. Biosynthesis and metabolism Glycerol 1-phosphate is synthesized by reducing dihydroxyacetone phosphate (DHAP), a glycolysis intermediate, with ''sn''-glycerol-1-phosphate dehydrogenase. DHAP and thus glycerol 1-phosphate is also possible to be synthesized from amino acids and citric acid cycle intermediates via gluconeogenesis pathway. : + NAD(P)H + H+ → + NAD(P)+ Glycerol 1-phosphate is a starting material for ''de novo'' synthesis of ether lipids, such as those derived from archaeol and caldarchaeol. It is first geranylgeranylated on its ''sn''-3 position by a cytosolic enzyme, phosphoglycerol geranylgeranyltransferase. A second geranylgeranyl group is then added on the ''sn''-2 position making unsaturated archaetidic acid. Enantiomer Organisms other than archaea, i.e. bacteria and eucarya, use the enantiomer, gly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionophore
In chemistry, an ionophore () is a chemical species that reversibly binds ions. Many ionophores are lipid-soluble entities that transport ions across the cell membrane. Ionophores catalyze ion transport across hydrophobic membranes, such as liquid polymeric membranes (carrier-based ion selective electrodes) or lipid bilayers found in the living cells or synthetic vesicles ( liposomes). Structurally, an ionophore contains a hydrophilic center and a hydrophobic portion that interacts with the membrane. Some ionophores are synthesized by microorganisms to import ions into their cells. Synthetic ion carriers have also been prepared. Ionophores selective for cations and anions have found many applications in analysis. These compounds have also shown to have various biological effects and a synergistic effect when combined with the ion they bind. Classification Biological activities of metal ion-binding compounds can be changed in response to the increment of the metal conce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylcholine
Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoyl phosphatidylcholine (a.k.a. lecithin) is a major component of pulmonary surfactant and is often used in the L/S ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria, including ''Escherichia coli''. Purified phosphatidylcholine is produced commercially. The name ''lecithin'' was derived from Greek λέκιθος, ''lekithos'' 'egg yolk' by Theodore Nicolas Gobley, a French chemist and pharmacist of the mid-19th century, who ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geranylgeranyl Pyrophosphate
Geranylgeranyl pyrophosphate is an intermediate in the biosynthesis of diterpenes and diterpenoids. It is also the precursor to carotenoids, gibberellins, tocopherols, and chlorophylls. It is also a precursor to geranylgeranylated proteins, which is its primary use in human cells. It is formed from farnesyl pyrophosphate by the addition of an isoprene unit from isopentenyl pyrophosphate. In ''Drosophila'', geranylgeranyl pyrophosphate is synthesised by HMG-CoA encoded by the Columbus gene. Geranylgeranyl pyrophosphate is utilised as a chemoattractant for migrating germ cells that have traversed the midgut epithelia. The attractant signal is produced at the gonadal precursors, directing the germ cells to these sites, where they will differentiate into eggs and spermatozoa (sperm). Related compounds * Farnesyl pyrophosphate * Geranylgeraniol * Geranyl pyrophosphate Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpeno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopentenyl Pyrophosphate
Isopentenyl pyrophosphate (IPP, isopentenyl diphosphate, or IDP) is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway (commonly called the mevalonate pathway) and in the ''non-mevalonate'' MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids. Biosynthesis IPP is formed from acetyl-CoA via the mevalonate pathway (the "upstream" part), and then is isomerized to dimethylallyl pyrophosphate by the enzyme isopentenyl pyrophosphate isomerase. IPP can be synthesised via an alternative non-mevalonate pathway of isoprenoid precursor biosynthesis, the MEP pathway, where it is formed from (''E'')-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) by the enzyme HMB-PP reductase (LytB, IspH). The MEP pathway is present in many bacteria, apicomplexan protozoa such as malaria parasites, and in the plastids of higher plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylallyl Pyrophosphate
Dimethylallyl pyrophosphate (DMAPP; or alternatively, dimethylallyl diphosphate (DMADP); also isoprenyl pyrophosphate) is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP. In the mevalonate pathway DMAPP is synthesised from mevalonic acid. In contrast, DMAPP is synthesised from HMBPP in the MEP pathway The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-''C''-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the is .... At present, it is believed that there is crossover between the two pathways in organisms that use both pathways to create terpenes and terpenoids, such as in plants ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
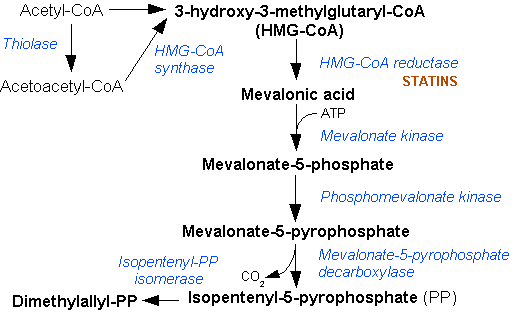
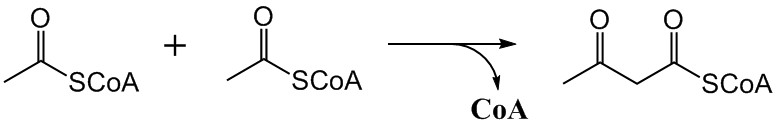
Mevalonic Acid Pathway
The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones. The mevalonate pathway begins with acetyl-CoA and ends with the production of IPP and DMAPP. It is best known as the target of statins, a class of cholesterol lowering drugs. Statins inhibit HMG-CoA reductase within the mevalonate pathway. Upper mevalonate pathway The mevalonate pathway of eukaryotes, archaea, and eubacteria all begin the same way. The sole carbon feed stock of the pathway is acetyl-CoA. The first step condenses two acetyl-CoA molecules to yield acetoacetyl-CoA. This is followed by a second condensation to form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

.jpg)