|
Aquadag
Aquadag is a trade name for a water-based colloidal graphite coating commonly used in cathode ray tubes (CRTs). It is manufactured by Acheson Industries, a subsidiary of ICI. The name is a shortened form of "Aqueous Deflocculated Acheson Graphite", but has become a generic term for conductive graphite coatings used in vacuum tubes. Other related products include Oildag, Electrodag and Molydag. Deflocculation refers to the distribution of powdered high purity graphite in an aqueous solution containing approximately 2% to 10% by weight of various Tannic/Gallotannic acid variants and separating the colloidal graphite suspension from the remaining unsuspended graphite particulates. The product names are often printed with DAG in upper case (e.g. AquaDAG). It is used as an electrically conductive coating on insulating surfaces, and as a lubricant. Properties Aquadag consists of a dispersion of colloidal graphite in distilled water. It is provided in concentrated paste form and is usu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathode Ray Tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), pictures (television set, computer monitor), radar targets, or other phenomena. A CRT on a television set is commonly called a picture tube. CRTs have also been used as memory devices, in which case the screen is not intended to be visible to an observer. The term ''cathode ray'' was used to describe electron beams when they were first discovered, before it was understood that what was emitted from the cathode was a beam of electrons. In CRT television sets and computer monitors, the entire front area of the tube is scanned repeatedly and systematically in a fixed pattern called a raster. In color devices, an image is produced by controlling the intensity of each of three electron beams, one for each additive primary color (red, green, and bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloidal
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word ''suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study was introduced in 1845 by Italian ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescent
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation. It is a form of luminescence. In most cases, the emitted light has a longer wavelength, and therefore a lower photon energy, than the absorbed radiation. A perceptible example of fluorescence occurs when the absorbed radiation is in the ultraviolet region of the electromagnetic spectrum (invisible to the human eye), while the emitted light is in the visible region; this gives the fluorescent substance a distinct color that can only be seen when the substance has been exposed to UV light. Fluorescent materials cease to glow nearly immediately when the radiation source stops, unlike phosphorescent materials, which continue to emit light for some time after. Fluorescence has many practical applications, including mineralogy, gemology, medicine, chemical sensors (fluorescence spectroscopy), fluorescent labelling, dyes, biological detectors, cosmic-ray detection, vacuu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
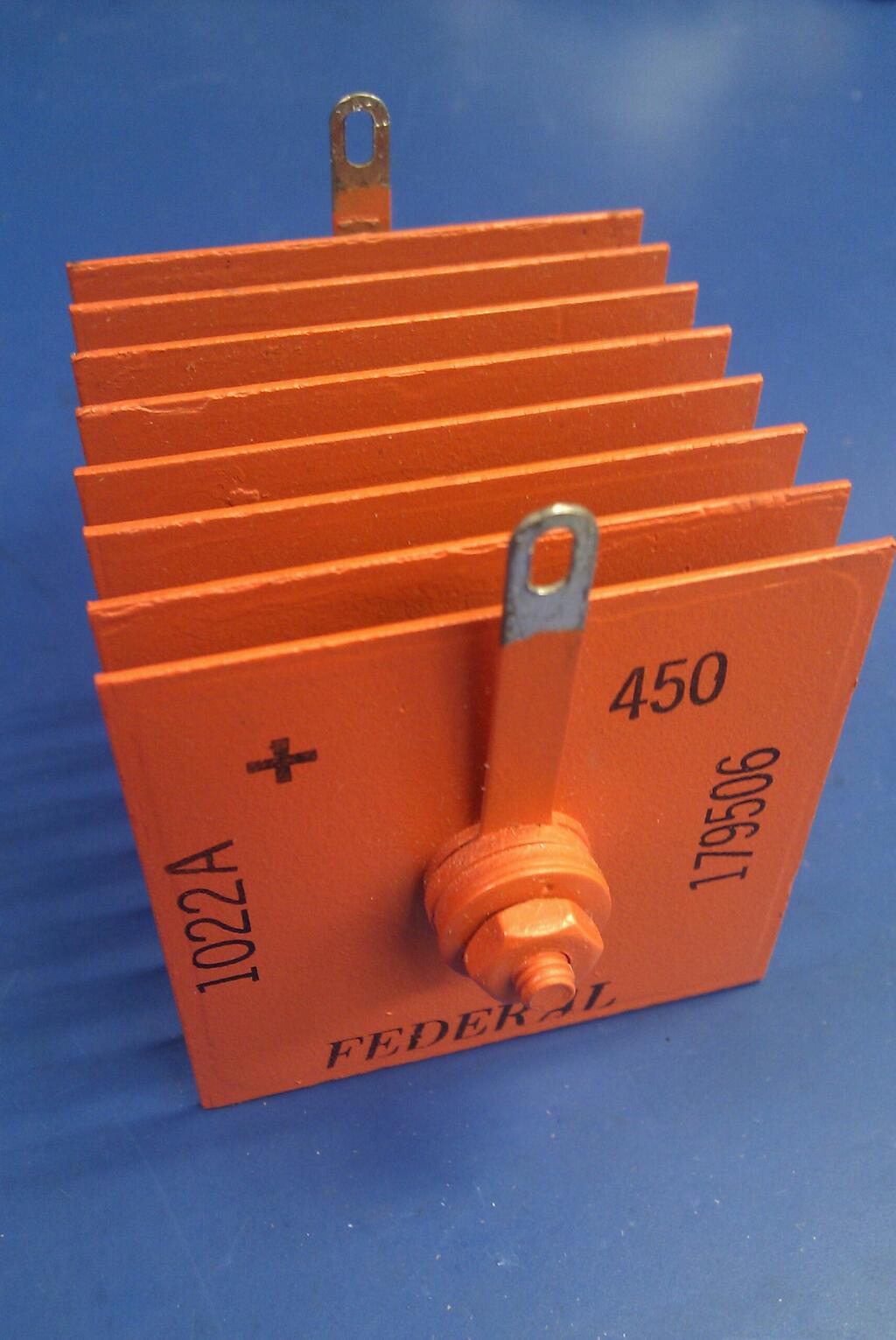
Metal Rectifier
A metal rectifier is an early type of semiconductor rectifier in which the semiconductor is copper oxide, germanium or selenium. They were used in power applications to convert alternating current to direct current in devices such as radios and battery chargers. Westinghouse Electric was a major manufacturer of these rectifiers since the late 1920s, under the trade name Westector (now used as a trade name for an overcurrent trip device by Westinghouse Nuclear). In some countries the term "metal rectifier" is applied to all such devices; in others the term "metal rectifier" normally refers to copper-oxide types, and "selenium rectifier" to selenium-iron types. Description Metal rectifiers consist of washer-like discs of different metals, either copper (with an oxide layer to provide the rectification) or steel or aluminium, plated with selenium. The discs are often separated by spacer sleeves to provide cooling. Mode of operation The principle of operation of a metal rect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Capacitor
A capacitor is a device that stores electrical energy in an electric field by virtue of accumulating electric charges on two close surfaces insulated from each other. It is a passive electronic component with two terminals. The effect of a capacitor is known as capacitance. While some capacitance exists between any two electrical conductors in proximity in a circuit, a capacitor is a component designed to add capacitance to a circuit. The capacitor was originally known as the condenser, a term still encountered in a few compound names, such as the ''condenser microphone''. The physical form and construction of practical capacitors vary widely and many types of capacitor are in common use. Most capacitors contain at least two electrical conductors often in the form of metallic plates or surfaces separated by a dielectric medium. A conductor may be a foil, thin film, sintered bead of metal, or an electrolyte. The nonconducting dielectric acts to increase the capacitor's c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dielectric
In electromagnetism, a dielectric (or dielectric medium) is an electrical insulator that can be polarised by an applied electric field. When a dielectric material is placed in an electric field, electric charges do not flow through the material as they do in an electrical conductor, because they have no loosely bound, or free, electrons that may drift through the material, but instead they shift, only slightly, from their average equilibrium positions, causing dielectric polarisation. Because of dielectric polarisation, positive charges are displaced in the direction of the field and negative charges shift in the direction opposite to the field (for example, if the field is moving parallel to the positive ''x'' axis, the negative charges will shift in the negative ''x'' direction). This creates an internal electric field that reduces the overall field within the dielectric itself. If a dielectric is composed of weakly bonded molecules, those molecules not only become polaris ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Filter Capacitor
Capacitors have many uses in electronic and electrical systems. They are so ubiquitous that it is rare that an electrical product does not include at least one for some purpose. Energy storage A capacitor can store electric energy when it is connected to its charging circuit and when it is disconnected from its charging circuit, it can dissipate that stored energy, so it can be used like a temporary battery. Capacitors are commonly used in electronic devices to maintain power supply while batteries are being changed. (This prevents loss of information in volatile memory.) Conventional electrostatic capacitors provide less than 360 joules per kilogram of energy density, while capacitors using developing technology can provide more than 2.52 kilo joules per kilogram. In car audio systems, large capacitors store energy for the amplifier to use on demand. An uninterruptible power supply (UPS) can be equipped with maintenance-free capacitors to extend service life. Pulsed powe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Secondary Electrons
Secondary electrons are electrons generated as ionization products. They are called 'secondary' because they are generated by other radiation (the ''primary'' radiation). This radiation can be in the form of ions, electrons, or photons with sufficiently high energy, i.e. exceeding the ionization potential. Photoelectrons can be considered an example of secondary electrons where the primary radiation are photons; in some discussions photoelectrons with higher energy (>50 eV) are still considered "primary" while the electrons freed by the photoelectrons are "secondary". Applications Secondary electrons are also the main means of viewing images in the scanning electron microscope (SEM). The range of secondary electrons depends on the energy. Plotting the inelastic mean free path as a function of energy often shows characteristics of the "universal curve" familiar to electron spectroscopists and surface analysts. This distance is on the order of a few nanometers in metals and t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or visible light, and cathodoluminescent substances which glow when struck by an electron beam (cathode rays) in a cathode-ray tube. When a phosphor is exposed to radiation, the orbital electrons in its molecules are excited to a higher energy level; when they return to their former level they emit the energy as light of a certain color. Phosphors can be classified into two categories: fluorescent substances which emit the energy immediately and stop glowing when the exciting radiation is turned off, and phosphorescent substances which emit the energy after a delay, so they keep glowing after the radiation is turned off, decaying in brightness over a period of milliseconds to days. Fluorescent materials are used in applications in which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Gun
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produces a narrow, collimated electron beam that has a precise kinetic energy. The largest use is in cathode-ray tubes (CRTs), used in nearly all television sets, computer displays and oscilloscopes that are not flat-panel displays. They are also used in field-emission displays (FEDs), which are essentially flat-panel displays made out of rows of extremely small cathode-ray tubes. They are also used in microwave linear beam vacuum tubes such as klystrons, inductive output tubes, travelling wave tubes, and gyrotrons, as well as in scientific instruments such as electron microscopes and particle accelerators. Electron guns may be classified by the type of electric field generation (DC or RF), by emission mechanism (thermionic, photocathode, cold emission, plasmas source), by focusing (pure electrostatic or with magnetic fields), or by the number of electrodes. Characteristics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on large scale (300 kton/year, in 1989) for uses in pencils, lubricants, and electrodes. Under high pressures and temperatures it converts to diamond. It is a weak conductor of heat and electricity. Types and varieties Natural graphite The principal types of natural graphite, each occurring in different types of ore deposits, are * Crystalline small flakes of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular; * Amorphous graphite: very fine flake graphite is sometimes called amorphous; * Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Beam
Cathode rays or electron beam (e-beam) are streams of electrons observed in discharge tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, glass behind the positive electrode is observed to glow, due to electrons emitted from the cathode (the electrode connected to the negative terminal of the voltage supply). They were first observed in 1859 by German physicist Julius Plücker and Johann Wilhelm Hittorf, and were named in 1876 by Eugen Goldstein ''Kathodenstrahlen'', or cathode rays. In 1897, British physicist J. J. Thomson showed that cathode rays were composed of a previously unknown negatively charged particle, which was later named the ''electron''. Cathode-ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to render an image on a screen. Description Cathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. To release electrons into the tube, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)




