|
Apamin
Apamin is an 18 amino acid globular peptide neurotoxin found in apitoxin ( bee venom). Dry bee venom consists of 2–3% of apamin. Apamin selectively blocks SK channels, a type of Ca2+-activated K+ channel expressed in the central nervous system. Toxicity is caused by only a few amino acids, in particular cysteine1, lysine4, arginine13, arginine14 and histidine18. These amino acids are involved in the binding of apamin to the Ca2+-activated K+ channel. Due to its specificity for SK channels, apamin is used as a drug in biomedical research to study the electrical properties of SK channels and their role in the afterhyperpolarizations occurring immediately following an action potential. Origin The first symptoms of apitoxin (bee venom), that are now thought to be caused by apamin, were described back in 1936 by Hahn and Leditschke. Apamin was first isolated by Habermann in 1965 from ''Apis mellifera'', the Western honey bee. Apamin was named after this bee. Bee venom contains many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tamapin
Tamapin is a toxin from the Indian Red Scorpion (''Hottentotta tamulus''), which is a selective and potent blocker of SK2 channels. Etymology Tamapin is named after the scorpion from which it was isolated. Sources Tamapin has been isolated from ''hottentotta tamulus'', the Indian red scorpion. Chemical structure and methods of isolation Tamapin belongs to short-chain scorpion toxin subfamily 5, together with PO5 and Scyllatoxin. Its sequence similarity to other toxins that can compete with the binding site of apamin is much lower. It is 31 amino acids long and its weight is 3458 Daltons. Its amino acid sequence is AFCNLRRCELSCRSLGLLGKCIGEECKCVPY, with disulfide bonds between Cys3-Cys21, Cys8-Cys26, and Cys12-Cys28 (chemical formula C146H234N42O42S6). Tamapin has been isolated via detection of the apamin-competing fraction of the venom from the scorpion via a Sephadex G-50 size exclusion chromatography, followed by high performance liquid chromatography (HPLC). An isoform ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SK Channel
SK channels (small conductance calcium-activated potassium channels) are a subfamily of calcium-activated potassium channels. They are so called because of their small single channel conductance in the order of 10 pS. SK channels are a type of ion channel allowing potassium cations to cross the cell membrane and are activated (opened) by an increase in the concentration of intracellular calcium through N-type calcium channels. Their activation limits the firing frequency of action potentials and is important for regulating afterhyperpolarization in the neurons of the central nervous system as well as many other types of electrically excitable cells. This is accomplished through the hyperpolarizing leak of positively charged potassium ions along their concentration gradient into the extracellular space. This hyperpolarization causes the membrane potential to become more negative. SK channels are thought to be involved in synaptic plasticity and therefore play important roles in l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
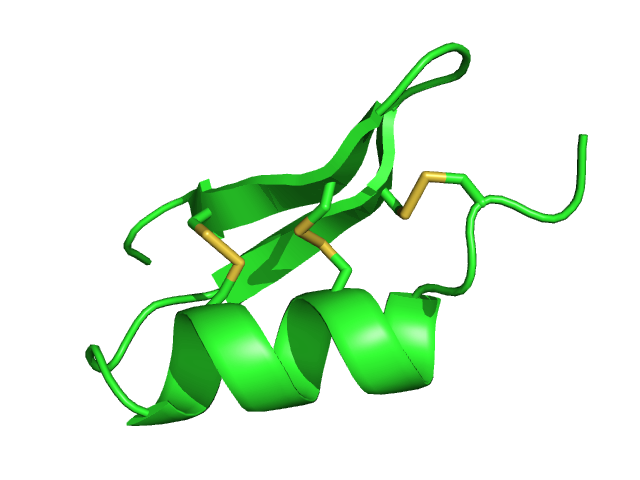
Scyllatoxin
Scyllatoxin (also leiurotoxin I) is a toxin, from the scorpion '' Leiurus quinquestriatus hebraeus'', which blocks small-conductance Ca2+-activated K+ channels. It is named after Scylla, a sea monster from Greek mythology. Charybdotoxin is also found in the venom from the same species of scorpion, and is named after the sea monster Charybdis. In Greek mythology, Scylla and Charybdis lived on rocks on opposing sides of a narrow strait of water. Sources Scyllatoxin is one of the components of the venom of the Israeli scorpion ''‘Leiurus quinquestriatus hebraeus’''. It consists of only 0.02% of the total protein in crude venom. Chemistry Leiurotoxin I is a 31-residue peptide (sequence AFCNLRMCQLSCRSLGLLGKCIGDKCECVKH-NH2), with a helix and a short antiparallel β-sheet. This toxin is stabilized by disulfide bonds: Cys8-Cys26 and Cys12-Cys28 is bound to the β-sheet, while Cys3-Cys21 is bound to an N-terminal segment preceding the helix. Leiurotoxin adopts the ά/β motif. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MCD Peptide
Mast cell degranulating (MCD) peptide is a cationic 22- amino acid residue peptide, which is a component of the venom of the bumblebee (''Megabombus pennsylvanicus''). At low concentrations, MCD peptide can stimulate mast cell degranulation. At higher concentrations, it has anti-inflammatory properties. In addition, it is a potent blocker of voltage-sensitive potassium channels. Sources MCD peptide is a component of bumblebee (''Megabombus pennsylvanicus'') venom. In addition to MCD peptide, melittin and apamin have also been identified in this venom and are also described as voltage-dependent channel blockers. MCD peptide is also present in the venom of the honey bee '' Apis mellifera''. Chemistry MCD peptide is a cationic 22-amino acid residue peptide with two disulfide bridges. Although the MCD peptide sequence shows similarity with apamin, they have different toxic properties. MCD peptide belongs to a large family composed of numerous derivatives detecting specific target ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Apitoxin
Apitoxin or bee venom is the venom produced by the honey bee. It is a cytotoxic and hemotoxic bitter colorless liquid containing proteins, which may produce local inflammation. It may have similarities to sea nettle toxin. Components Bee venom is a complex mixture of proteins and smaller molecules. The main component is melittin, which amounts to 52% of venom peptides One of the main allergens is phospholipase A2, which amounts to 12% and is an enzyme that catalyzes the hydrolysis of phospholipids, causing degradation of cell membranes. Adolapin contributes 2–5% of the peptides. Further protein components include apamin (2%), a neurotoxin, hyaluronidase (2%), which dilates blood vessels, increasing their permeability and facilitating the spread of the venom, mast cell degranulating peptide (2%), tertiapin, and secapin. Small molecules in bee venom include histamine (0.1–1%), dopamine and noradrenaline. Research Apitoxins are under preliminary research for their potent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FT-IR
Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared Electromagnetic spectrum, spectrum of Absorption (electromagnetic radiation), absorption or Emission (electromagnetic radiation), emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a Dispersion (optics), dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time. The term ''Fourier-transform infrared spectroscopy'' originates from the fact that a Fourier transform (a mathematical process) is required to convert the raw data into the actual spectrum. Conceptual introduction The goal of absorption spectroscopy techniques (FTIR, Ultraviolet-visible spectroscopy, ultraviolet-visible ("UV-vis") spectroscopy, etc.) is to measure how much light a sample absorbs at each wavelength. The most straightforward way to do this, the "dispe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acids
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,2-Cyclohexanedione
1,2-Cyclohexanedione is an organic compound with the formula (CH)(CO). It is one of three isomeric cyclohexanediones. It is a colorless compound that is soluble in a variety of organic solvents. It can be prepared by oxidation of cyclohexanone by selenium dioxide. The enol is about 1 kcal/mol more stable than the diketo form. Numerous diimine and dioxime ligands have been prepared from this diketone. It condenses with 1,2-diamine A diamine is an amine with exactly two amino groups. Diamines are used as monomers to prepare polyamides, polyimides, and polyureas. The term ''diamine'' refers mostly to primary diamines, as those are the most reactive. In terms of quantities p ...s to give diaza heterocycles. References {{DEFAULTSORT:Cyclohexanedione, 1, 2- Diketones Cyclic ketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trypsin
Trypsin is an enzyme in the first section of the small intestine that starts the digestion of protein molecules by cutting these long chains of amino acids into smaller pieces. It is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated. Trypsin cuts peptide chains mainly at the carboxyl side of the amino acids lysine or arginine. It is used for numerous biotechnological processes. The process is commonly referred to as trypsin proteolysis or trypsinization, and proteins that have been digested/treated with trypsin are said to have been trypsinized. Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Function In the duodenum, trypsin catalyzes the hydrolysis of pept ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PubChem
PubChem is a database of chemical molecules and their activities against biological assays. The system is maintained by the National Center for Biotechnology Information (NCBI), a component of the National Library of Medicine, which is part of the United States National Institutes of Health (NIH). PubChem can be accessed for free through a web user interface. Millions of compound structures and descriptive datasets can be freely downloaded via FTP. PubChem contains multiple substance descriptions and small molecules with fewer than 100 atoms and 1,000 bonds. More than 80 database vendors contribute to the growing PubChem database. History PubChem was released in 2004 as a component of the Molecular Libraries Program (MLP) of the NIH. As of November 2015, PubChem contains more than 150 million depositor-provided substance descriptions, 60 million unique chemical structures, and 225 million biological activity test results (from over 1 million assay experiments performed on more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calmodulin
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases. Structure Calmodulin is a small, highly conserved protein that is 148 amino acids long (16.7 kDa). The protein has two approximately symmetrical globular domains (the N- and C- domains) each containing a pair of EF hand motifs separated by a flexible linker region for a total of four Ca2+ binding sites, two in each globular domain. In the Ca2+-free state, the helices that form the four EF-hands are collapsed in a compact orientation, and the central linker is disordered; in the Ca2+-saturated state, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |