|
Ammonium Acetate
Ammonium acetate, also known as spirit of Mindererus in aqueous solution, is a chemical compound with the formula NH4CH3CO2. It is a white, hygroscopic solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially. Uses It is the main precursor to acetamide: :NH4CH3CO2 → CH3C(O)NH2 + H2O It is also used as a diuretic. Buffer As the salt of a weak acid and a weak base, ammonium acetate is often used with acetic acid to create a buffer solution. Ammonium acetate is volatile at low pressures. Because of this, it has been used to replace cell buffers that contain non-volatile salts in preparing samples for mass spectrometry. It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate. When dissolving ammonium acetate in pure water, the resulting solution typically has a pH of 7, because the equal amounts of acetate and ammonium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deliquescent
Hygroscopy is the phenomenon of attracting and holding water molecules via either absorption or adsorption from the surrounding environment, which is usually at normal or room temperature. If water molecules become suspended among the substance's molecules, adsorbing substances can become physically changed, e.g., changing in volume, boiling point, viscosity or some other physical characteristic or property of the substance. For example, a finely dispersed hygroscopic powder, such as a salt, may become clumpy over time due to collection of moisture from the surrounding environment. ''Deliquescent'' materials are sufficiently hygroscopic that they absorb so much water that they become liquid and form an aqueous solution. Etymology and pronunciation The word ''hygroscopy'' () uses combining forms of '' hygro-'' and '' -scopy''. Unlike any other ''-scopy'' word, it no longer refers to a viewing or imaging mode. It did begin that way, with the word ''hygroscope'' referring in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
High-performance Liquid Chromatography
High-performance liquid chromatography (HPLC), formerly referred to as high-pressure liquid chromatography, is a technique in analytical chemistry used to separate, identify, and quantify each component in a mixture. It relies on pumps to pass a pressurized liquid solvent containing the sample mixture through a column filled with a solid adsorbent material. Each component in the sample interacts slightly differently with the adsorbent material, causing different flow rates for the different components and leading to the separation of the components as they flow out of the column. HPLC has been used for manufacturing (''e.g.'', during the production process of pharmaceutical and biological products), legal (''e.g.'', detecting performance enhancement drugs in urine), research (''e.g.'', separating the components of a complex biological sample, or of similar synthetic chemicals from each other), and medical (''e.g.'', detecting vitamin D levels in blood serum) purposes. Chrom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Carbonate
Ammonium carbonate is a salt with the chemical formula (NH4)2CO3. Since it readily degrades to gaseous ammonia and carbon dioxide upon heating, it is used as a leavening agent and also as smelling salt. It is also known as baker's ammonia and is a predecessor to the more modern leavening agents baking soda and baking powder. It is a component of what was formerly known as sal volatile and salt of hartshorn, and produces a pungent smell when baked. Production Ammonium carbonate is produced by combining carbon dioxide and aqueous ammonia. About 80,000 tons/year were produced as of 1997. An orthorhombic monohydrate is known. It crystallizes in an ammonia solution exposed in a carbon dioxide-rich atmosphere. Decomposition Ammonium carbonate slowly decomposes at standard temperature and pressure through two pathways. Thus any initially pure sample of ammonium carbonate will soon become a mixture including various byproducts. Ammonium carbonate can spontaneously decompose into ammo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
INS Number
The International Numbering System for Food Additives (INS) is a European-based naming system for food additives, aimed at providing a short designation of what may be a lengthy actual name."Class Names and the International Numbering System for Food Additives"CAC/GL 36-1989 Adopted in 1989, Revision 2008. Last amendment 2011. Published by Codex Alimentarius It is defined by Codex Alimentarius, the international food standards organisation of the World Health Organization (WHO) and Food and Agriculture Organization (FAO) of the United Nations (UN). The information is published in the document ''Class Names and the International Numbering System for Food Additives'', first published in 1989, with revisions in 2008 and 2011. The INS is an open list, "subject to the inclusion of additional additives or removal of existing ones on an ongoing basis". Numbering system INS numbers consist of three or four digits, optionally followed by an alphabetical suffix to further characterize indiv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food Additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives have been used for centuries as part of an effort to preserve food, for example vinegar (pickling), salt (salting), smoke (smoking), sugar (crystallization), etc. This allows for longer-lasting foods such as bacon, sweets or wines. With the advent of processed foods in the second half of the twentieth century, many additives have been introduced, of both natural and artificial origin. Food additives also include substances that may be introduced to food indirectly (called "indirect additives") in the manufacturing process, through packaging, or during storage or transport. Numbering To regulate these additives and inform consumers, each additive is assigned a unique number called an "E number", which is used in Europe for all approved additives. This numbering scheme has now been adopted and extended by the '' Codex Alimentarius'' Commission to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation Exchange Capacity
Cation-exchange capacity (CEC) is a measure of how many cations can be retained on soil particle surfaces. Negative charges on the surfaces of soil particles bind positively-charged atoms or molecules (cations), but allow these to exchange with other positively charged particles in the surrounding soil water. This is one of the ways that solid materials in soil alter the chemistry of the soil. CEC affects many aspects of soil chemistry, and is used as a measure of soil fertility, as it indicates the capacity of the soil to retain several nutrients (e.g. K+, NH4+, Ca2+) in plant-available form. It also indicates the capacity to retain pollutant cations (e.g. Pb2+). Definition and principles Cation-exchange capacity is defined as the amount of positive charge that can be exchanged per mass of soil, usually measured in cmolc/kg. Some texts use the older, equivalent units me/100g or meq/100g. CEC is measured in moles of electric charge, so a cation-exchange capacity of 10 cmolc/kg coul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
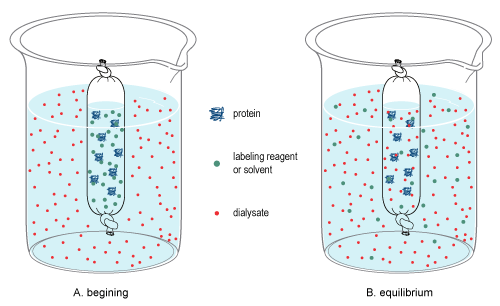
Dialysis (biochemistry)
In chemistry, dialysis is the process of separating molecules in Solution (chemistry), solution by the difference in their rates of diffusion through a semipermeable membrane, such as dialysis tubing. Dialysis is a common laboratory technique that operates on the same principle as Kidney_dialysis, medical dialysis. In the context of life science research, the most common application of dialysis is for the removal of unwanted small molecules such as salts, reducing agents, or dyes from larger macromolecules such as proteins, DNA, or polysaccharides. Dialysis is also commonly used for buffer exchange and drug binding studies. The concept of dialysis was introduced in 1861 by the Scottish chemist Thomas Graham (chemist), Thomas Graham. He used this technique to separate sucrose (small molecule) and gum Arabic solutes (large molecule) in aqueous solution. He called the diffusible solutes crystalloids and those that would not pass the membrane colloids. From this concept dialysis ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one of the most important branches of organic chemistry. There are several main areas of research within the general area of organic synthesis: ''total synthesis'', ''semisynthesis'', and ''methodology''. Total synthesis A total synthesis is the complete chemical synthesis of complex organic molecules from simple, commercially available petrochemical or natural precursors. Total synthesis may be accomplished either via a linear or convergent approach. In a ''linear'' synthesis—often adequate for simple structures—several steps are performed one after another until the molecule is complete; the chemical compounds made in each step are called synthetic intermediates. Most often, each step in a synthesis refers to a separate rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borch Reaction
Sodium cyanoborohydride is the chemical compound with the formula Na B H3 CN. It is a colourless salt, but commercial samples can appear tan. It is widely used in organic synthesis for the reduction of imines. The salt tolerates aqueous conditions. Use Owing to the presence of the electron-withdrawing cyanide substituent, (CN)H3sup>− is less reducing than is −.html" ;"title="H4sup>−">H4sup>−. As a mild reducing agent, it is used to convert imines to amines. It is especially favored for reductive aminations, wherein aldehydes or ketones are treated with an amine in the presence of this reagent: : R2CO + R'NH2 + NaBH3CN + CH3OH → R2CH-NHR' + "NaCH3OBH2CN" The reagent is typically used in excess. Selectivity is achieved at mildly basic solutions ( pH 7–10). The reagent is ideal for reductive aminations ("Borch Reaction"). In conjunction with tosylhydrazine, sodium cyanoborohydride is used in the reductive deoxygenation of ketones. Structure and prepar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
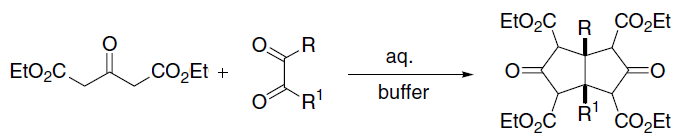
Knoevenagel Condensation
In organic chemistry, the Knoevenagel condensation () reaction is a type of chemical reaction named after German chemist Emil Knoevenagel. It is a modification of the aldol condensation. A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence ''condensation''). The product is often an α,β-unsaturated ketone (a conjugated enone). In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form * or for instance diethyl malonate, Meldrum's acid, ethyl acetoacetate or malonic acid, or cyanoacetic acid. * , for instance nitromethane. where Z is an electron withdrawing group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensation of the aldehyde o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonium Formate
Ammonium formate, NH4HCO2, is the ammonium salt of formic acid. It is a colorless, hygroscopic, crystalline solid. Reductive amination Acetone can be transformed into isopropylamine as follows: :CH3C(O)CH3 + 2 HCO2− +NH4 → (CH3)2CHNHCHO + 2 H2O + NH3 + CO2 :(CH3)2CHNHCHO + H2O → (CH3)2CHNH2 + HCO2H Uses Pure ammonium formate decomposes into formamide and water when heated, and this is its primary use in industry. Formic acid can also be obtained by reacting ammonium formate with a dilute acid, and since ammonium formate is also produced from formic acid, it can serve as a way of storing formic acid. Ammonium formate can also be used in palladium on carbon (Pd/C) reduction of functional groups. In the presence of Pd/C, ammonium formate decomposes to hydrogen, carbon dioxide, and ammonia. This hydrogen gas is adsorbed onto the surface of the palladium metal, where it can react with various functional groups. For example, alkenes can be reduced to alkanes, formaldehyde to met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporative Light Scattering Detector
An evaporative light scattering detector (ELSD) is a detector used in conjunction with high-performance liquid chromatography (HPLC), Ultra high-performance liquid chromatography (UHPLC), Purification liquid chromatography such as flash or preparative chromatography, countercurrent or centrifugal partition chromatographies and Supercritical Fluid chromatography (SFC). It is commonly used for analysis of compounds where UV detection might be a restriction and therefore used where compounds do not efficiently absorb UV radiation, such as sugars, antivirals, antibiotics, fatty acids, lipids, oils, phospholipids, polymers, surfactants, terpenoids and triglycerides. ELSDs is related to the Charged aerosol detector, charged aerosol detector (CAD) and like the CAD, falls under the category of destructive detectors. An evaporative light scattering detector (ELSD) is able to detect all compound which are less volatile than the mobile phase, i.e. non volatile and semi-volatile compounds. Pri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |