|
Allylmagnesium Bromide
Allylmagnesium bromide is a Grignard reagent used for introducing the allyl group. It is commonly available as a solution in diethyl ether. It may be synthesized by treatment of magnesium with allyl bromide Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and p ... while maintaining the reaction temperature below 0 °C to suppress formation of hexadiene. Allyl chloride can also be used in place of the bromide to give allylmagnesium chloride. These reagents are used to prepare metal allyl complexes. References Further reading * {{cite book , author = Chabot, P. , editor1=Rakita, P. E. , editor2=Silverman, G. , chapter = 7. Infrared and Raman Spectroscopy , title = Handbook of Grignard Reagents , year = 1996 , pages = 93–102 , location = New York, N.Y. , publisher = Marcel Dekke ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
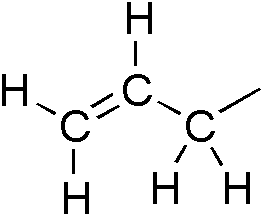
Allyl Group
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diethyl Ether
Diethyl ether, or simply ether, is an organic compound in the ether class with the formula , sometimes abbreviated as (see Pseudoelement symbols). It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It is commonly used as a solvent in laboratories and as a starting fluid for some engines. It was formerly used as a general anesthetic, until non-flammable drugs were developed, such as halothane. It has been used as a recreational drug to cause intoxication. Production Most diethyl ether is produced as a byproduct of the vapor-phase hydration of ethylene to make ethanol. This process uses solid-supported phosphoric acid catalysts and can be adjusted to make more ether if the need arises. Vapor-phase dehydration of ethanol over some alumina catalysts can give diethyl ether yields of up to 95%. Diethyl ether can be prepared both in laboratories and on an industrial scale by the acid ether synthesis. Ethanol is mixed with a stro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Bromide
Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, synthetic perfumes and other organic compounds. Physically, allyl bromide is a colorless liquid with an irritating and persistent smell, however, commercial samples are yellow or brown. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use. Preparation Allyl bromide is produced commercially from allyl alcohol and hydrobromic acid: :CH2=CHCH2OH + HBr → CH2=CHCH2Br + H2O It can also be prepared by the halogen-exchange reaction between allyl chloride and hydrobromic acid or by the allylic bromination of propene. Reactions and uses Electrophilic properties Allyl bromide is an electrophilic alkylating agent. It reacts with nucleophiles, such as amines, carbanions, alkoxides, etc., to introduce the allyl group: :CH2=CHCH2Br + Nu− → CH2=CHCH2Nu + Br− (Nu− is a nucleophile) It is used in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transition-metal Allyl Complex
Transition-metal allyl complexes are coordination complexes with allyl and its derivatives as ligands. Allyl is the radical with the connectivity CH2CHCH2, although as a ligand it is usually viewed as an allyl anion CH2=CH−CH2−, which is usually described as two equivalent resonance structures. Examples The allyl ligand is commonly found in organometallic chemistry. Most commonly, allyl ligands bind to metals via all three carbon atoms, the η3-binding mode. The η3-allyl group is classified as an LX-type ligand in the Green LXZ ligand classification scheme, serving as a 3e– donor using neutral electron counting and 4e– donor using ionic electron counting. More common are complexes with allyl and other ligands. Examples include (η3-allyl)Mn(CO)4 and CpPd(allyl). Homoleptic complexes * bis(allyl)nickel * bis(allyl)palladium * bis(allyl)platinum *tris(allyl)chromium * tris(allyl)rhodium * tris(allyl)iridium Synthetic methods Allyl complexes are often generated by ox ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organomagnesium Compounds
Magnesium anthracenide with three thf ligands. Group 2 organometallic chemistry refers to the chemistry of compounds containing carbon bonded to any group 2 element. By far the most common group 2 organometallic compounds are the magnesium-containing Grignard reagents which are widely used in organic chemistry. Other organmetallic group 2 compounds are rare and are typically limited to academic interests. Characteristics As the group 2 elements (also referred to as the alkaline earth metals) contain two valence electrons, their chemistries have similarities group 12 organometallic compounds. Both readily assume a +2 oxidation states with higher and lower states being rare, and are less electronegative than carbon. However, as the group two elements (with the exception of beryllium) have considerably low electronegativity the resulting C-M bonds are more highly polarized and ionic-like, if not entirely ionic for the heavier barium compounds. The lighter organoberyllium and orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allyl Compounds
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated an allyl derivative from garlic oil and named it "". The term allyl applies to many compounds related to , some of which are of practical or of everyday importance, for example, allyl chloride. Allylation is any chemical reaction that adds an allyl group to a substrate. Nomenclature A site adjacent to the unsaturated carbon atom is called the allylic position or allylic site. A group attached at this site is sometimes described as allylic. Thus, "has an allylic hydroxyl group". Allylic C−H bonds are about 15% weaker than the C−H bonds in ordinary sp3 carbon centers and are thus more reactive. Benzylic and allylic are related in terms of structure, bond strength, and reactivity. Other re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |