|
Allotropes Of Iron
At atmospheric pressure, three allotropic forms of iron exist, depending on temperature: alpha iron (α-Fe), gamma iron (γ-Fe), and delta iron (δ-Fe). At very high pressure, a fourth form exists, called epsilon iron (ε-Fe). Some controversial experimental evidence suggests the existence of a fifth high-pressure form that is stable at very high pressures and temperatures. The phases of iron at atmospheric pressure are important because of the differences in solubility of carbon, forming different types of steel. The high-pressure phases of iron are important as models for the solid parts of planetary cores. The inner core of the Earth is generally assumed to consist essentially of a crystalline iron-nickel alloy with ε structure. The outer core surrounding the solid inner core is believed to be composed of liquid iron mixed with nickel and trace amounts of lighter elements. Standard pressure allotropes Alpha iron (α-Fe) Below 912 °C (1,674 °F), iron has a b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
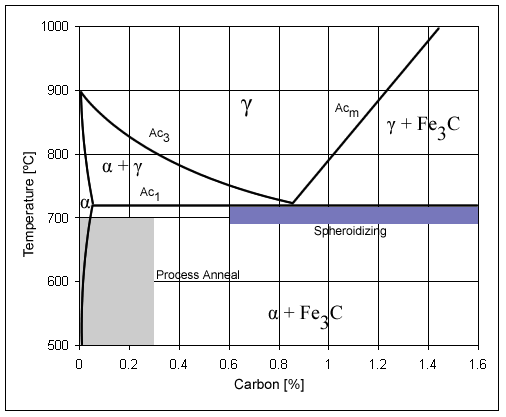
Pure Iron Phase Diagram (EN)
Pure may refer to: Computing * A pure function * A pure virtual function * PureSystems, a family of computer systems introduced by IBM in 2012 * Pure Software, a company founded in 1991 by Reed Hastings to support the Purify tool * Pure-FTPd, FTP server software * Pure (programming language), functional programming language based on term rewriting * Pure Storage, a company that makes datacenter storage solutions * Pure (CRIS), a research information system bought by Elsevier. Companies and products * Pure (app), dating app * Pure (restaurant chain), a British fast food chain * Pure Insurance, Privilege Underwriters Reciprocal Exchange * Pure Trading, a Canadian electronic communication network operated by CNQ * Pure Digital, a UK consumer electronics company specialising in DAB radios * Pure Oil, a U.S. chain of gas stations * Propulsion Universelle et Récuperation d'Énergie (PURE), a motorsport engineering company * Pure FM (Portsmouth), a university radio station based in P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Paramagnetism
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, diamagnetic materials are repelled by magnetic fields and form induced magnetic fields in the direction opposite to that of the applied magnetic field. Paramagnetic materials include most chemical elements and some compounds; they have a relative magnetic permeability slightly greater than 1 (i.e., a small positive magnetic susceptibility) and hence are attracted to magnetic fields. The magnetic moment induced by the applied field is linear in the field strength and rather weak. It typically requires a sensitive analytical balance to detect the effect and modern measurements on paramagnetic materials are often conducted with a SQUID magnetometer. Paramagnetism is due to the presence of unpaired electrons in the material, so most atoms w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Phys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Brinell Scale
The Brinell scale characterizes the indentation hardness of materials through the scale of penetration of an indenter, loaded on a material test-piece. It is one of several definitions of hardness in materials science. History Proposed by Swedish engineer Johan August Brinell in 1900, it was the first widely used and standardised hardness test in engineering and metallurgy. The large size of indentation and possible damage to test-piece limits its usefulness. However, it also had the useful feature that the hardness value divided by two gave the approximate UTS in ksi for steels. This feature contributed to its early adoption over competing hardness tests. Test details The typical test uses a diameter steel ball as an indenter with a force. For softer materials, a smaller force is used; for harder materials, a tungsten carbide ball is substituted for the steel ball. The indentation is measured and hardness calculated as: :\operatorname=\frac where: :BHN = Brinell Hard ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferromagnetic
Ferromagnetism is a property of certain materials (such as iron) which results in a large observed magnetic permeability, and in many cases a large magnetic coercivity allowing the material to form a permanent magnet. Ferromagnetic materials are the familiar metals noticeably attracted to a magnet, a consequence of their large magnetic permeability. Magnetic permeability describes the induced magnetization of a material due to the presence of an ''external'' magnetic field, and it is this temporarily induced magnetization inside a steel plate, for instance, which accounts for its attraction to the permanent magnet. Whether or not that steel plate acquires a permanent magnetization itself, depends not only on the strength of the applied field, but on the so-called coercivity of that material, which varies greatly among ferromagnetic materials. In physics, several different types of material magnetism are distinguished. Ferromagnetism (along with the similar effect ferrimagnet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cast Iron
Cast iron is a class of iron– carbon alloys with a carbon content more than 2%. Its usefulness derives from its relatively low melting temperature. The alloy constituents affect its color when fractured: white cast iron has carbide impurities which allow cracks to pass straight through, grey cast iron has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and ductile cast iron has spherical graphite "nodules" which stop the crack from further progressing. Carbon (C), ranging from 1.8 to 4 wt%, and silicon (Si), 1–3 wt%, are the main alloying elements of cast iron. Iron alloys with lower carbon content are known as steel. Cast iron tends to be brittle, except for malleable cast irons. With its relatively low melting point, good fluidity, castability, excellent machinability, resistance to deformation and wear resistance, cast irons have become an engineering material with a wide range of applicatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mild Steel
Carbon steel is a steel with carbon content from about 0.05 up to 2.1 percent by weight. The definition of carbon steel from the American Iron and Steel Institute (AISI) states: * no minimum content is specified or required for chromium, cobalt, molybdenum, nickel, niobium, titanium, tungsten, vanadium, zirconium, or any other element to be added to obtain a desired alloying effect; * the specified minimum for copper does not exceed 0.40%; * or the maximum content specified for any of the following elements does not exceed the percentages noted: manganese 1.65%; silicon 0.60%; copper 0.60%. The term ''carbon steel'' may also be used in reference to steel which is not stainless steel; in this use carbon steel may include alloy steels. High carbon steel has many different uses such as milling machines, cutting tools (such as chisels) and high strength wires. These applications require a much finer microstructure, which improves the toughness. Carbon steel is a popular metal cho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples of physical properties include density, index of refraction, magnetization and chemical composition. A simple description is that a phase is a region of material that is chemically uniform, physically distinct, and (often) mechanically separable. In a system consisting of ice and water in a glass jar, the ice cubes are one phase, the water is a second phase, and the humid air is a third phase over the ice and water. The glass of the jar is another separate phase. (See ) The term ''phase'' is sometimes used as a synonym for state of matter, but there can be several immiscible phases of the same state of matter. Also, the term ''phase'' is sometimes used to refer to a set of equilibrium states demarcated in terms of state variables such as pressure and temperature by a phase boundary on a phase diagram. Beca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cementite
Cementite (or iron carbide) is a compound of iron and carbon, more precisely an intermediate transition metal carbide with the formula Fe3C. By weight, it is 6.67% carbon and 93.3% iron. It has an orthorhombic crystal structure. It is a hard, brittle material, normally classified as a ceramic in its pure form, and is a frequently found and important constituent in ferrous metallurgy. While cementite is present in most steels and cast irons, it is produced as a raw material in the iron carbide process, which belongs to the family of alternative ironmaking technologies. The name ''cementite'' originated from the theory of Floris Osmond and J. Werth, in which the structure of solidified steel consists of a kind of cellular tissue, with ferrite as the nucleus and Fe3C the envelope of the cells. The carbide therefore ''cemented'' the iron. Metallurgy In the iron–carbon system (i.e. plain-carbon steels and cast irons) it is a common constituent because ferrite can contain at most ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Eutectoid
A eutectic system or eutectic mixture ( ) is a homogeneous mixture that has a melting point lower than those of the constituents. The lowest possible melting point over all of the mixing ratios of the constituents is called the ''eutectic temperature''. On a phase diagram, the eutectic temperature is seen as the eutectic point (see plot on the right). Non-eutectic mixture ratios would have different melting temperatures for their different constituents, since one component's lattice will melt at a lower temperature than the other's. Conversely, as a non-eutectic mixture cools down, each of its components would solidify (form a lattice) at a different temperature, until the entire mass is solid. Not all binary alloys have eutectic points, since the valence electrons of the component species are not always compatible, in any mixing ratio, to form a new type of joint crystal lattice. For example, in the silver-gold system the melt temperature (liquidus) and freeze temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase Diagram
A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium. Overview Common components of a phase diagram are ''lines of equilibrium'' or ''phase boundaries'', which refer to lines that mark conditions under which multiple phases can coexist at equilibrium. Phase transitions occur along lines of equilibrium. Metastable phases are not shown in phase diagrams as, despite their common occurrence, they are not equilibrium phases. Triple points are points on phase diagrams where lines of equilibrium intersect. Triple points mark conditions at which three different phases can coexist. For example, the water phase diagram has a triple point corresponding to the single temperature and pressure at which solid, liquid, and gaseous water can coexist in a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |