|
Aerogel
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. The first documented example of an aerogel was created by Samuel Stephens Kistler in 1931, as a result of a bet with Charles Learned over who could replace the liquid in "jellies" with gas without causing shrinkage. Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The first aer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aerogel Hand
Aerogels are a class of synthetic porous ultralight material derived from a gel, in which the liquid component for the gel has been replaced with a gas, without significant collapse of the gel structure. The result is a solid with extremely low density and extremely low thermal conductivity. Aerogels can be made from a variety of chemical compounds. Silica aerogels feel like fragile expanded polystyrene to the touch, while some polymer-based aerogels feel like rigid foams. The first documented example of an aerogel was created by Samuel Stephens Kistler in 1931, as a result of a bet with Charles Learned over who could replace the liquid in "jellies" with gas without causing shrinkage. Aerogels are produced by extracting the liquid component of a gel through supercritical drying or freeze-drying. This allows the liquid to be slowly dried off without causing the solid matrix in the gel to collapse from capillary action, as would happen with conventional evaporation. The first aer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ultralight Material
Ultralight materials are solids with a density of less than 10 mg/cm3, including silica aerogels, carbon nanotube aerogels, aerographite, metallic foams, polymeric foams, and metallic microlattices. The density of air is about 1.275 mg/cm3, which means that the air in the pores contributes significantly to the density of these materials in atmospheric conditions. They can be classified by production method as aerogels, stochastic foams, and structured cellular materials. Properties Ultralight materials are solids with a density of less than 10 mg/cm3. Ultralight material is defined by its cellular arrangement and its stiffness and strength that make up its solid constituent. They include silica aerogels, carbon nanotube aerogels, aero graphite, metallic foams, polymeric foams, and metallic micro lattices. Ultralight materials are produced to have the strength of bulk-scaled properties at a micro-size. Also, they are designed to not compress even under extreme pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Samuel Stephens Kistler
Samuel Stephens Kistler (March 26, 1900 November 6, 1975) was an American scientist and chemical engineer, best known as the inventor of aerogels, one of the lightest known solid materials. Biography Kistler, the son of a shopkeeper, was born in the small town of Cedarville in the far northeastern corner of California. The family moved to the larger Santa Rosa when Kistler was 12, where he first became interested in chemistry. When he entered the College of the Pacific in 1917, however, his plan was to learn to play the cello, then pursue a degree in agriculture. Instead, he ended up taking every science course available, and after three years he moved to Stanford University and obtained a B.A. in chemistry, followed by a chemical engineering degree. He never did learn to play the cello. After a brief spell working for the Standard Oil Company of California, he returned to academia, teaching chemistry at the College of the Pacific until 1931, when he transferred to the Universit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermal Insulation
Thermal insulation is the reduction of heat transfer (i.e., the transfer of thermal energy between objects of differing temperature) between objects in thermal contact or in range of radiative influence. Thermal insulation can be achieved with specially engineered methods or processes, as well as with suitable object shapes and materials. Heat flow is an inevitable consequence of contact between objects of different temperature. Thermal insulation provides a region of insulation in which thermal conduction is reduced, creating a thermal break or thermal barrier, or thermal radiation is reflected rather than absorbed by the lower-temperature body. The insulating capability of a material is measured as the inverse of thermal conductivity (k). Low thermal conductivity is equivalent to high insulating capability ( resistance value). In thermal engineering, other important properties of insulating materials are product density (ρ) and specific heat capacity (c). Definition T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically, density is defined as mass divided by volume: : \rho = \frac where ''ρ'' is the density, ''m'' is the mass, and ''V'' is the volume. In some cases (for instance, in the United States oil and gas industry), density is loosely defined as its weight per unit volume, although this is scientifically inaccurate – this quantity is more specifically called specific weight. For a pure substance the density has the same numerical value as its mass concentration. Different materials usually have different densities, and density may be relevant to buoyancy, purity and packaging. Osmium and iridium are the densest known elements at standard conditions for temperature and pressure. To simplify comparisons of density across different s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Friability
Friability ( ), the condition of being friable, describes the tendency of a solid substance to break into smaller pieces under duress or contact, especially by rubbing. The opposite of friable is indurate. Substances that are designated hazardous, such as asbestos or crystalline silica, are often said to be friable if small particles are easily dislodged and become airborne, and hence respirable (able to enter human lungs), thereby posing a health hazard. Tougher substances, such as concrete, may also be mechanically ground down and reduced to finely divided mineral dust. However, such substances are not generally considered friable because of the degree of difficulty involved in breaking the substance's chemical bonds through mechanical means. Some substances, such as polyurethane foams, show an increase in friability with exposure to ultraviolet radiation, as in sunlight. Friable is sometimes used metaphorically to describe "brittle" personalities who can be "rubbed" by seemingl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
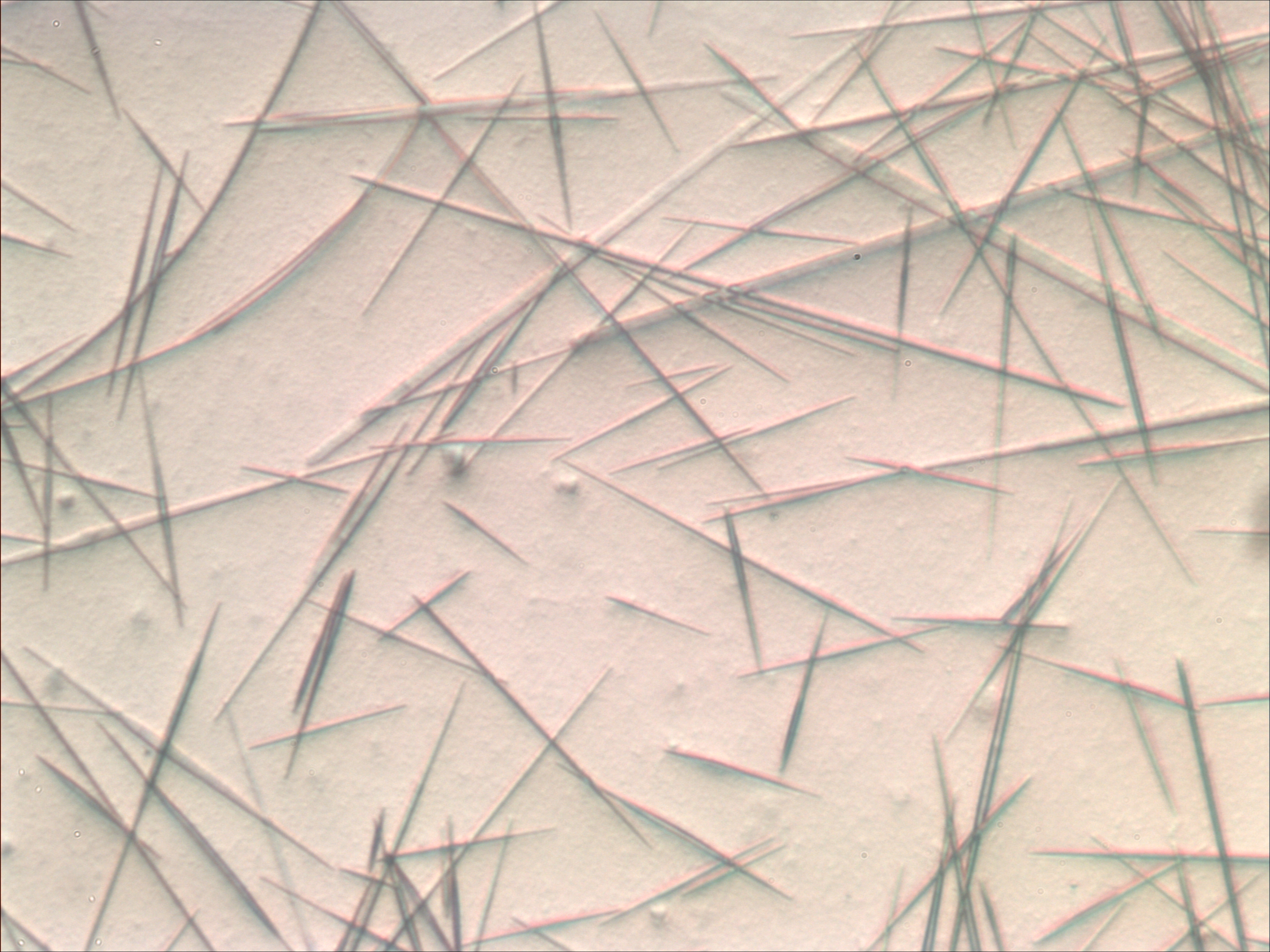
Dendrite (metal)
A dendrite in metallurgy is a characteristic tree-like structure of crystals growing as molten metal solidifies, the shape produced by faster growth along energetically favourable crystallographic directions. This dendritic growth has large consequences in regard to material properties. Dendrites form in unary (one-component) systems as well as multi-component systems. The requirement is that the liquid (the molten material) be undercooled, aka supercooled, below the freezing point of the solid. Initially, a spherical solid nucleus grows in the undercooled melt. As the sphere grows, the spherical morphology becomes unstable and its shape becomes perturbed. The solid shape begins to express the preferred growth directions of the crystal. This growth direction may be due to anisotropy in the surface energy of the solid–liquid interface, or to the ease of attachment of atoms to the interface on different crystallographic planes, or both (for an example of the latter, see hopp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin Dioxide
Tin(IV) oxide, also known as stannic oxide, is the inorganic compound with the formula SnO2. The mineral form of SnO2 is called cassiterite, and this is the main ore of tin. With many other names, this oxide of tin is an important material in tin chemistry. It is a colourless, diamagnetic, amphoteric solid. Structure Tin(IV) oxide crystallises with the rutile structure. As such the tin atoms are six coordinate and the oxygen atoms three coordinate. SnO2 is usually regarded as an oxygen-deficient n-type semiconductor. Hydrous forms of SnO2 have been described as stannic acid. Such materials appear to be hydrated particles of SnO2 where the composition reflects the particle size. Preparation Tin(IV) oxide occurs naturally. Synthetic tin(IV) oxide is produced by burning tin metal in air. Annual production is in the range of 10 kilotons. SnO2 is reduced industrially to the metal with carbon in a reverberatory furnace at 1200–1300 °C. Amphoterism Although SnO2 is insoluble ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Description The combining capacity, or affinity of an ...—its atom making four electrons available to form covalent bond, covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon makes up only about 0.025 percent of Earth's crust. Three Isotopes of carbon, isotopes occur naturally, Carbon-12, C and Carbon-13, C being stable, while Carbon-14, C is a radionuclide, decaying with a half-life of about 5,730 years. Carbon is one of the Timeline of chemical element discoveries#Ancient discoveries, few elements known since antiquity. Carbon is the 15th Abundance of elements in Earth's crust, most abundant element in the Earth's crust, and the Abundance of the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nanometre
330px, Different lengths as in respect to the molecular scale. The nanometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: nm) or nanometer (American and British English spelling differences#-re, -er, American spelling) is a units of measurement, unit of length in the International System of Units (SI), equal to one billionth (short scale) of a metre () and to 1000 picometres. One nanometre can be expressed in scientific notation as , and as metres. History The nanometre was formerly known as the millimicrometre – or, more commonly, the millimicron for short – since it is of a micron (micrometre), and was often denoted by the symbol mμ or (more rarely and confusingly, since it logically should refer to a ''millionth'' of a micron) as μμ. Etymology The name combines the SI prefix ''nano-'' (from the Ancient Greek , ', "dwarf") with the parent unit name ''metre'' (from Greek , ', "unit of measurement"). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spherical
A sphere () is a geometrical object that is a three-dimensional analogue to a two-dimensional circle. A sphere is the set of points that are all at the same distance from a given point in three-dimensional space.. That given point is the centre of the sphere, and is the sphere's radius. The earliest known mentions of spheres appear in the work of the ancient Greek mathematicians. The sphere is a fundamental object in many fields of mathematics. Spheres and nearly-spherical shapes also appear in nature and industry. Bubbles such as soap bubbles take a spherical shape in equilibrium. The Earth is often approximated as a sphere in geography, and the celestial sphere is an important concept in astronomy. Manufactured items including pressure vessels and most curved mirrors and lenses are based on spheres. Spheres roll smoothly in any direction, so most balls used in sports and toys are spherical, as are ball bearings. Basic terminology As mentioned earlier is the sphere's r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |