|
Activator (proteomics)
Enzyme activators are molecules that bind to enzymes and increase their activity. They are the opposite of enzyme inhibitors. These molecules are often involved in the allosteric regulation of enzymes in the control of metabolism. An example of an enzyme activator working in this way is fructose 2,6-bisphosphate, which activates phosphofructokinase 1 and increases the rate of glycolysis in response to the hormone glucagon. In some cases, when a substrate binds to one catalytic subunit of an enzyme, this can trigger an increase in the substrate affinity as well as catalytic activity in the enzyme's other subunits, and thus the substrate acts as an activator. Examples Hexokinase-I Hexokinase-I (HK-I) is an enzyme activator because it draws glucose into the glycolysis pathway. Its function is to phosphorylate glucose releasing glucose-6-phosphate (G6P) as the product. HK-I not only signals the activation of glucose into glycolysis but also maintains a low glucose concentration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphofructokinase 6PFK Wpmp
Phosphofructokinase (PFK) is a kinase enzyme that phosphorylation, phosphorylates fructose 6-phosphate in glycolysis. Function The enzyme-catalysed transfer of a phosphoryl group from Adenosine triphosphate, ATP is an important reaction in a wide variety of biological processes. Phosphofructokinase catalyses the phosphorylation of fructose-6-phosphate to fructose 1,6-bisphosphate, fructose-1,6-bisphosphate, a key regulatory step in the glycolytic pathway. It is Allosteric regulation, allosterically inhibited by ATP and allosterically activated by Adenosine monophosphate, AMP, thus indicating the cell's energetic needs when it undergoes the glycolytic pathway. PFK exists as a homotetramer in bacteria and Mammal, mammals (where each monomer possesses 2 similar Protein domain, domains) and as an octomer in yeast (where there are 4 alpha- (PFK1) and 4 beta-chains (PFK2), the latter, like the mammalian monomers, possessing 2 similar domains). This protein may use the morpheein model ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Activity
Enzyme assays are laboratory methods for measuring enzymatic activity. They are vital for the study of enzyme kinetics and enzyme inhibition. Enzyme units The quantity or concentration of an enzyme can be expressed in molar amounts, as with any other chemical, or in terms of activity in enzyme units. Enzyme activity Enzyme activity is a measure of the quantity of active enzyme present and is thus dependent on various physical conditions, ''which should be specified''. It is calculated using the following formula: :\operatorname=\operatorname=\operatorname\times\operatorname where :\operatorname= Enzyme activity :\operatorname= Moles of substrate converted per unit time :\operatorname= Rate of the reaction :\operatorname= Reaction volume The SI unit is the katal, 1 katal = 1 mol s−1 (mole per second), but this is an excessively large unit. A more practical and commonly used value is enzyme unit (U) = 1 μmol min−1 (micromole per minute). 1 U corresponds to 16.67 nano ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme Inhibitor
An enzyme inhibitor is a molecule that binds to an enzyme and blocks its activity. Enzymes are proteins that speed up chemical reactions necessary for life, in which substrate molecules are converted into products. An enzyme facilitates a specific chemical reaction by binding the substrate to its active site, a specialized area on the enzyme that accelerates the most difficult step of the reaction. An enzyme inhibitor stops ("inhibits") this process, either by binding to the enzyme's active site (thus preventing the substrate itself from binding) or by binding to another site on the enzyme such that the enzyme's catalysis of the reaction is blocked. Enzyme inhibitors may bind reversibly or irreversibly. Irreversible inhibitors form a chemical bond with the enzyme such that the enzyme is inhibited until the chemical bond is broken. By contrast, reversible inhibitors bind non-covalently and may spontaneously leave the enzyme, allowing the enzyme to resume its function. Reve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Allosteric Regulation
In biochemistry, allosteric regulation (or allosteric control) is the regulation of an enzyme by binding an effector molecule at a site other than the enzyme's active site. The site to which the effector binds is termed the ''allosteric site'' or ''regulatory site''. Allosteric sites allow effectors to bind to the protein, often resulting in a conformational change and/or a change in protein dynamics. Effectors that enhance the protein's activity are referred to as ''allosteric activators'', whereas those that decrease the protein's activity are called ''allosteric inhibitors''. Allosteric regulations are a natural example of control loops, such as feedback from downstream products or feedforward from upstream substrates. Long-range allostery is especially important in cell signaling. Allosteric regulation is also particularly important in the cell's ability to adjust enzyme activity. The term ''allostery'' comes from the Ancient Greek ''allos'' (), "other", and ''stereos' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metabolism
Metabolism (, from el, μεταβολή ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the conversion of food to building blocks for proteins, lipids, nucleic acids, and some carbohydrates; and the elimination of metabolic wastes. These enzyme-catalyzed reactions allow organisms to grow and reproduce, maintain their structures, and respond to their environments. The word metabolism can also refer to the sum of all chemical reactions that occur in living organisms, including digestion and the transportation of substances into and between different cells, in which case the above described set of reactions within the cells is called intermediary (or intermediate) metabolism. Metabolic reactions may be categorized as ''catabolic'' – the ''breaking down'' of compounds (for example, of glucose to pyruvate by ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fructose 2,6-bisphosphate
Fructose 2,6-bisphosphate, abbreviated Fru-2,6-''P''2, is a metabolite that allosterically affects the activity of the enzymes phosphofructokinase 1 (PFK-1) and fructose 1,6-bisphosphatase (FBPase-1) to regulate glycolysis and gluconeogenesis. Fru-2,6-''P''2 itself is synthesized and broken down by the bifunctional enzyme phosphofructokinase 2/ fructose-2,6-bisphosphatase (PFK-2/FBPase-2). The synthesis of Fru-2,6-''P''2 is performed through a bifunctional enzyme containing both PFK-2 and FBPase-2, which is dephosphorylated, allowing the PFK-2 portion to phosphorylate fructose 6-phosphate using ATP. The breakdown of Fru-2,6-''P''2 is catalyzed by the phosphorylation of the bifunctional enzyme, which allows FBPase-2 to dephosphorylate fructose 2,6-bisphosphate to produce fructose 6-phosphate and Pi. Effects on glucose metabolism Fru-2,6-''P''2 strongly activates glucose breakdown in glycolysis through allosteric modulation (activation) of phosphofructokinase 1 (PFK-1). Elevate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphofructokinase 1
Phosphofructokinase-1 (PFK-1) is one of the most important regulatory enzymes () of glycolysis. It is an allosteric enzyme made of 4 subunits and controlled by many activators and inhibitors. PFK-1 catalyzes the important "committed" step of glycolysis, the conversion of fructose 6-phosphate and ATP to fructose 1,6-bisphosphate and ADP. Glycolysis is the foundation for respiration, both anaerobic and aerobic. Because phosphofructokinase (PFK) catalyzes the ATP-dependent phosphorylation to convert fructose-6-phosphate into fructose 1,6-bisphosphate and ADP, it is one of the key regulatory steps of glycolysis. PFK is able to regulate glycolysis through allosteric inhibition, and in this way, the cell can increase or decrease the rate of glycolysis in response to the cell's energy requirements. For example, a high ratio of ATP to ADP will inhibit PFK and glycolysis. The key difference between the regulation of PFK in eukaryotes and prokaryotes is that in eukaryotes PFK is acti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolysis
Glycolysis is the metabolic pathway that converts glucose () into pyruvate (). The free energy released in this process is used to form the high-energy molecules adenosine triphosphate (ATP) and reduced nicotinamide adenine dinucleotide (NADH). Glycolysis is a sequence of ten reactions catalyzed by enzymes. Glycolysis is a metabolic pathway that does not require oxygen (In anaerobic conditions pyruvate is converted to lactic acid). The wide occurrence of glycolysis in other species indicates that it is an ancient metabolic pathway. Indeed, the reactions that make up glycolysis and its parallel pathway, the pentose phosphate pathway, occur in the oxygen-free conditions of the Archean oceans, also in the absence of enzymes, catalyzed by metal. In most organisms, glycolysis occurs in the liquid part of cells, the cytosol. The most common type of glycolysis is the ''Embden–Meyerhof–Parnas (EMP) pathway'', which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucagon
Glucagon is a peptide hormone, produced by alpha cells of the pancreas. It raises concentration of glucose and fatty acids in the bloodstream, and is considered to be the main catabolic hormone of the body. It is also used as a Glucagon (medication), medication to treat a number of health conditions. Its effect is opposite to that of insulin, which lowers extracellular glucose. It is produced from proglucagon, encoded by the ''GCG'' gene. The pancreas releases glucagon when the amount of glucose in the bloodstream is too low. Glucagon causes the liver to engage in glycogenolysis: converting stored glycogen into glucose, which is released into the bloodstream. High blood-glucose levels, on the other hand, stimulate the release of insulin. Insulin allows glucose to be taken up and used by insulin-dependent tissues. Thus, glucagon and insulin are part of a feedback system that keeps blood glucose levels stable. Glucagon increases energy expenditure and is elevated under conditions of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
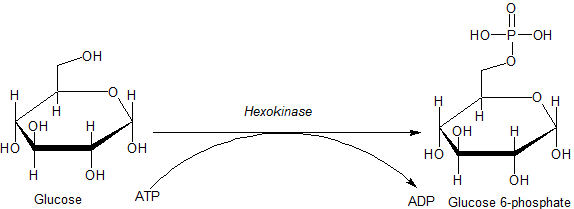
Hexokinase
A hexokinase is an enzyme that phosphorylates hexoses (six-carbon sugars), forming hexose phosphate. In most organisms, glucose is the most important substrate for hexokinases, and glucose-6-phosphate is the most important product. Hexokinase possesses the ability to transfer an inorganic phosphate group from ATP to a substrate. Hexokinases should not be confused with glucokinase, which is a specific isoform of hexokinase. All hexokinases are capable of phosphorylating several hexoses but glucokinase acts with a 50-fold lower substrate affinity and its main hexose substrate is glucose. Variation Genes that encode hexokinase have been discovered in every domain of life, and exist among a variety of species that range from bacteria, yeast, and plants to humans and other vertebrates. They are categorized as ''actin fold'' proteins, sharing a common ATP binding site core that is surrounded by more variable sequences which determine substrate affinities and other properties. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose-6-phosphate
Glucose 6-phosphate (G6P, sometimes called the Robison ester) is a glucose sugar phosphorylated at the hydroxy group on carbon 6. This dianion is very common in cells as the majority of glucose entering a cell will become phosphorylated in this way. Because of its prominent position in cellular chemistry, glucose 6-phosphate has many possible fates within the cell. It lies at the start of two major metabolic pathways: glycolysis and the pentose phosphate pathway. In addition to these two metabolic pathways, glucose 6-phosphate may also be converted to glycogen or starch for storage. This storage is in the liver and muscles in the form of glycogen for most multicellular animals, and in intracellular starch or glycogen granules for most other organisms. Production From glucose Within a cell, glucose 6-phosphate is produced by phosphorylation of glucose on the sixth carbon. This is catalyzed by the enzyme hexokinase in most cells, and, in higher animals, glucokinase in certain cells ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |